Question: What would differ if we used LDA as a base in this synthesis? Explain. What would differ if we used LDA as a base and
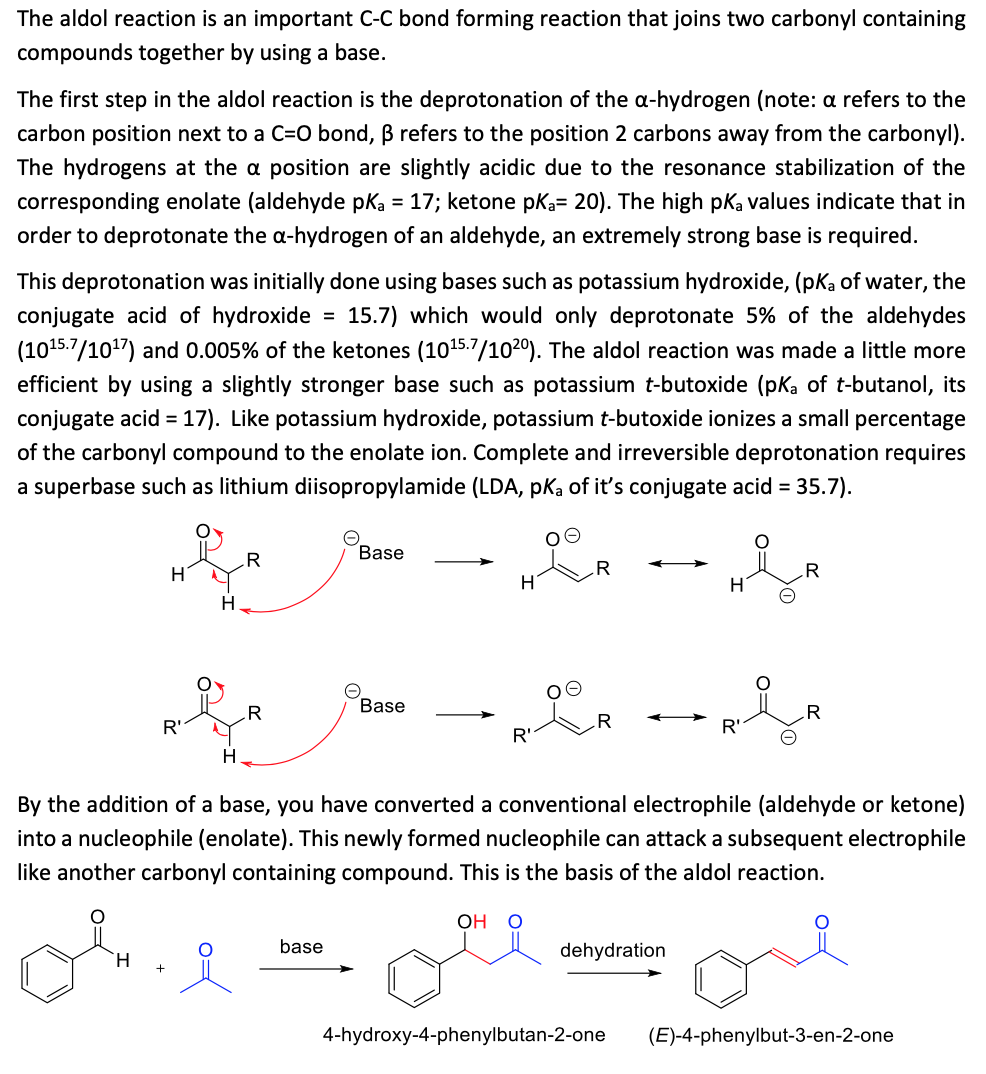
The aldol reaction is an important C-C bond forming reaction that joins two carbonyl containing compounds together by using a base. The first step in the aldol reaction is the deprotonation of the a-hydrogen (note: a refers to the carbon position next to a C=0 bond, B refers to the position 2 carbons away from the carbonyl). The hydrogens at the a position are slightly acidic due to the resonance stabilization of the corresponding enolate (aldehyde pka = 17; ketone pka= 20). The high pka values indicate that in order to deprotonate the a-hydrogen of an aldehyde, an extremely strong base is required. This deprotonation was initially done using bases such as potassium hydroxide, (pKa of water, the conjugate acid of hydroxide = 15.7) which would only deprotonate 5% of the aldehydes (1015.7/1017) and 0.005% of the ketones (1015.7/1020). The aldol reaction was made a little more efficient by using a slightly stronger base such as potassium t-butoxide (pk of t-butanol, its conjugate acid = 17). Like potassium hydroxide, potassium t-butoxide ionizes a small percentage of the carbonyl compound to the enolate ion. Complete and irreversible deprotonation requires a superbase such as lithium diisopropylamide (LDA, pKa of it's conjugate acid = 35.7). Base .R .R H Base .R R' R' H. By the addition of a base, you have converted a conventional electrophile (aldehyde or ketone) into a nucleophile (enolate). This newly formed nucleophile can attack a subsequent electrophile like another carbonyl containing compound. This is the basis of the aldol reaction. base dehydration H. 4-hydroxy-4-phenylbutan-2-one (E)-4-phenylbut-3-en-2-one
Step by Step Solution
3.53 Rating (160 Votes )
There are 3 Steps involved in it
Lithium Diisopropyl Amide LDA is a Strong Bulky base It forms Eno... View full answer

Get step-by-step solutions from verified subject matter experts


