Question: Which combination(s) of solutions result in a buffer? Select all that apply. A solution of NH3(aq) and a solution of HCl(aq) that contain equal numbers
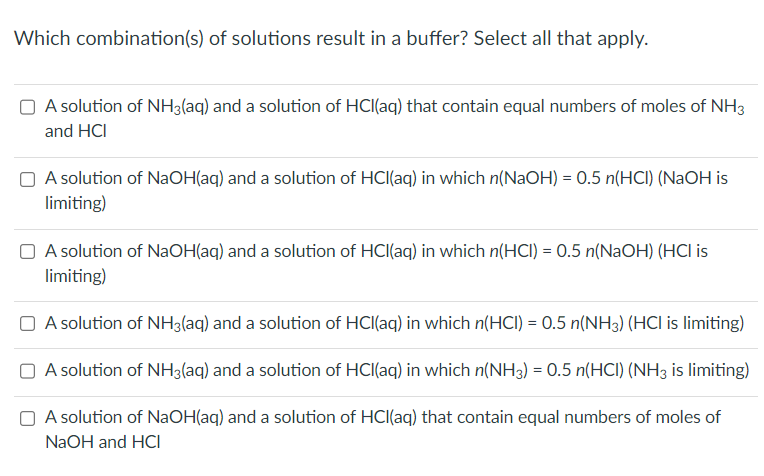
Which combination(s) of solutions result in a buffer? Select all that apply. A solution of NH3(aq) and a solution of HCl(aq) that contain equal numbers of moles of NH3 and HCl A solution of NaOH(aq) and a solution of HCl(aq) in which n(NaOH)=0.5n(HCl)(NaOH is limiting) A solution of NaOH(aq) and a solution of HCl(aq) in which n(HCl)=0.5n(NaOH)(HCl is limiting) A solution of NH3(aq) and a solution of HCl(aq) in which n(HCl)=0.5n(NH3)(HCl is limiting) A solution of NH3(aq) and a solution of HCl(aq) in which n(NH3)=0.5n(HCl)(NH3 is limiting) A solution of NaOH(aq) and a solution of HCl(aq) that contain equal numbers of moles of NaOH and HCl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


