Question: Consider the reaction: 2 SO2(g) + O2(g) 2 SO3ls) at constant temperature. Initially a container is filled with pure SO3(s) at a pressure of
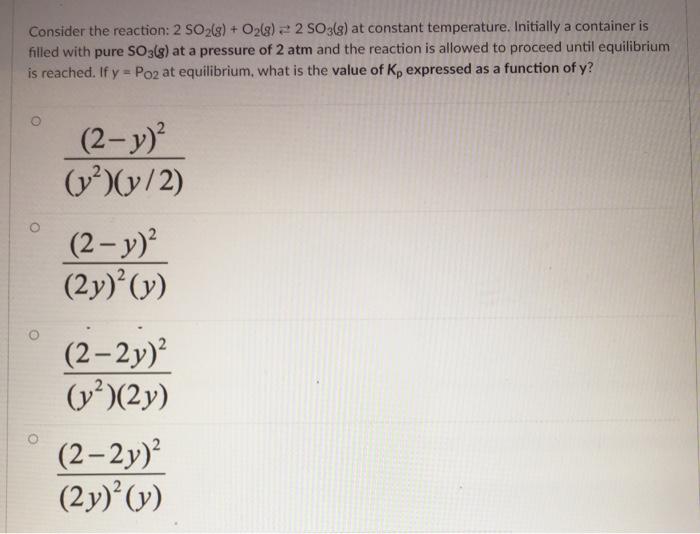
Consider the reaction: 2 SO2(g) + O2(g) 2 SO3ls) at constant temperature. Initially a container is filled with pure SO3(s) at a pressure of 2 atm and the reaction is allowed to proceed until equilibrium is reached. If y = Po2 at equilibrium, what is the value of Kp expressed as a function of y? (2-y) (v)(y/2) (2- y) (2y) (y) (2-2) (v)(2y) (2-2y) (2y) (y)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


