Many transuranium elements, such as plutonium-232, have very short half-lives. (For 232 Pu, the half-life is 36
Question:
Many transuranium elements, such as plutonium-232, have very short half-lives. (For 232Pu, the half-life is 36 minutes.) However, some, like protactinium-231 (half-life = 3.34 × 104 years), have relatively long half-lives. Use the masses given in the following table to calculate the change in energy when 1 mole of 232Pu nuclei and 1 mole of 231Pa nuclei are each formed from their respective number of protons and neutrons.
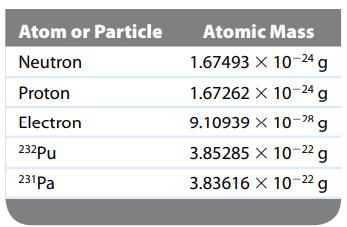
(Since the masses of 232Pu and 231Pa are atomic masses, they each include the mass of the electrons present. The mass of the nucleus will be the atomic mass minus the mass of the electrons.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:





