Question: Write the balanced formula, complete ionic, and net ionic equations for each of the following acidbase reactions. a. HNO3(aq) + Al(OH)3(s) b. HCHO(aq) + KOH(aq)
Write the balanced formula, complete ionic, and net ionic equations for each of the following acid–base reactions.
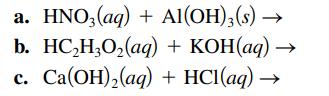
a. HNO3(aq) + Al(OH)3(s) b. HCHO(aq) + KOH(aq) C. Ca(OH)(aq) + HCl(aq)
Step by Step Solution
3.40 Rating (166 Votes )
There are 3 Steps involved in it
a HNO3aq AlOH3s AlNO33aq H2Ol Complete ionic equation H... View full answer

Get step-by-step solutions from verified subject matter experts


