Question: Using the data given in Example 15.2, calculate [N 2 O 5 ] 150. s after the start of the reaction. Data from Example 15.2
Using the data given in Example 15.2, calculate [N2O5] 150. s after the start of the reaction.
Data from Example 15.2
The decomposition of N2O5 in the gas phase was studied at constant temperature:
![]()
The following results were collected:
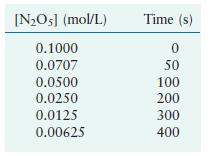
Using these data, verify that the rate law is first order in [N2O5], and calculate the value of the rate constant, where the rate = –d[N2O5]/dt.
2NOs(g) 4NO(g) + O(g)
Step by Step Solution
3.40 Rating (156 Votes )
There are 3 Steps involved in it
We know from Example 152 that NO5 00500 molL at 100 s and NO... View full answer

Get step-by-step solutions from verified subject matter experts


