Question: Using data in Appendix 2B, calculate the standard potential for the half-reaction Ti 4+ (aq) + 4 e Ti(s). 2B STANDARD POTENTIALS AT
Using data in Appendix 2B, calculate the standard potential for the half-reaction Ti4+(aq) + 4 e– → Ti(s).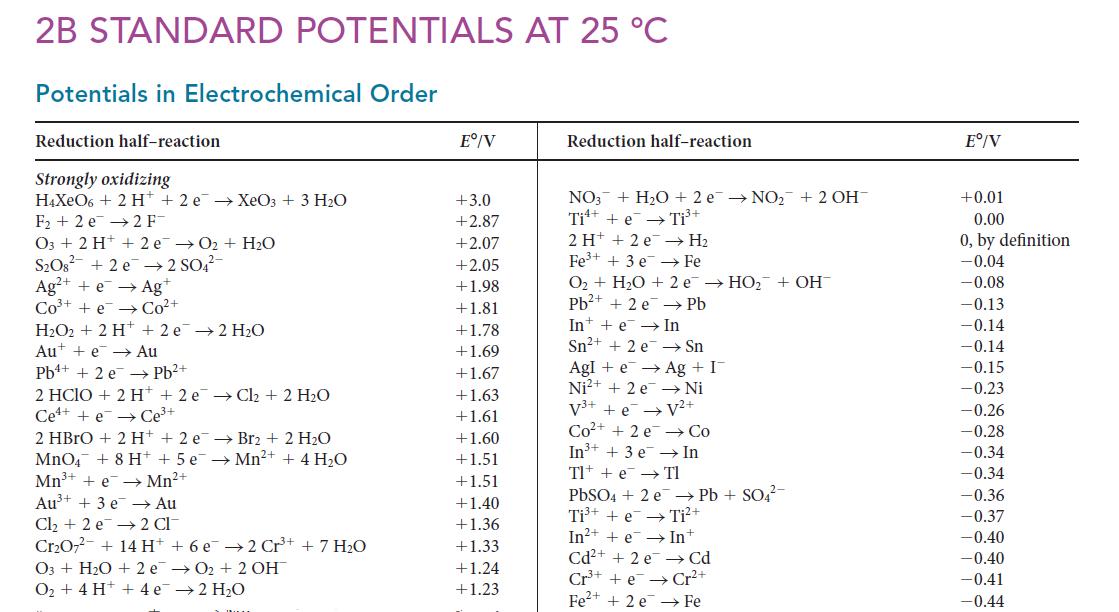
2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+ 2 e XeO3 + 3 HO F2 e 2 F- O3 + 2 H+ 2 e 0 + HO SO8 +2e 2 SO4- Ag+ + e Ag+ CO+ +e Co+ HO2 + 2 H + 2e 2 H0 Aue Au Pb+ Pb + 2 e 2 HCIO + 2 H+2 e Cl + 2 HO Cee Ce+ 2 HBrO + 2 H+ + 2 e Br2 + 2 HO MnO4 + 8 H+ + 5 e Mn+ + 4HO Mn+ + e Mn+ 2 CI Au+ + 3 e Au Cl + 2 e CrO7 + 14 H O3+ HO + 2e O + 4 H+ + 4e + 6 e2 Cr+ + 7 HO O + 2 OH 2HO E/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 +1.51 +1.51 +1.40 +1.36 +1.33 +1.24 +1.23 Reduction half-reaction NO3 + HO + 2 e NO + 2 OH Ti + e Ti+ 2H+ +2 e Fe+ + 3 e H Fe O + HO + 2e HO + OH Pb+ + 2 e Pb In e In Sn+ + 2 e Sn AgI+eAg + I Ni2+ + 2e Ni V+ + e V+ Co+ +2e Co In+ + 3 e In Tl + e Tl PbSO4 + 2 e Pb + SO4- Ti+ + e Ti+ In++eIn+ Cd+ + 2 e Cd Cr+e Cr+ Fe+ + 2 e Fe E/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40 -0.41 -0.44
Step by Step Solution
3.52 Rating (182 Votes )
There are 3 Steps involved in it
The appropriate halfreactions Ti e Ti E 000 A Ti e Ti E 037 B Ti 2e Ti E 163 C A B and ... View full answer

Get step-by-step solutions from verified subject matter experts


