Question: Using only the periodic table, predict the most stable ion for (mathrm{Na}, mathrm{Mg}, mathrm{Al}, mathrm{S}, mathrm{Cl}, mathrm{K}, mathrm{Ca}), and (mathrm{Ga}). Arrange these from largest to
Using only the periodic table, predict the most stable ion for \(\mathrm{Na}, \mathrm{Mg}, \mathrm{Al}, \mathrm{S}, \mathrm{Cl}, \mathrm{K}, \mathrm{Ca}\), and \(\mathrm{Ga}\). Arrange these from largest to smallest radius and explain why the radius varies as it does. Compare your predictions with Fig. 13.8.
Figure 13.8
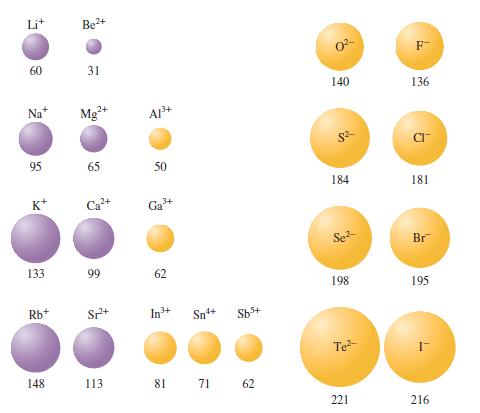
60 Na* 95 K* 133 Rb+ Be+ 31 Mg+ 65 A1+ Sp+ 50 3+ Ga 99 62 In+ Sn4+ Sb+ 148 113 81 71 62 0- 140 $2 184 Se- 198 Te- 221 F- 136 CI- 181 Br 195 216
Step by Step Solution
3.26 Rating (152 Votes )
There are 3 Steps involved in it
Na Sodium Predicted most stable ion Na loses one electron to achieve a noble gas configuration Larger radius than Na Already provided Explanation The ... View full answer

Get step-by-step solutions from verified subject matter experts


