Question: Using the solubility rules in Table 4.1 as a guide, predict whether an insoluble product forms when each of the following pairs of solutions is
Using the solubility rules in Table 4.1 as a guide, predict whether an insoluble product forms when each of the following pairs of solutions is mixed. Write the balanced chemical equation if a precipitation reaction does occur.
(a) Pb(NO3)2 and sodium carbonate
(b) Ammonium bromide and AgClO4
(c) Potassium hydroxide and copper(II) chloride
(d) Aammonium bromide and cobalt(II) sulfate
Strategy
Write the formulas of the new potential ionic compounds by making a table of cations and anions and their combinations. Use the solubility rules to determine whether any of the combinations are insoluble.
Table 4.1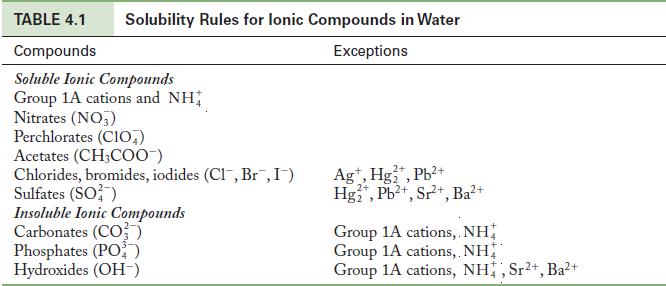
TABLE 4.1 Compounds Soluble Ionic Compounds Group 1A cations and NH Nitrates (NO3) Solubility Rules for lonic Compounds in Water Exceptions Perchlorates (CIO) Acetates (CH3COO-) Chlorides, bromides, iodides (Cl, Br, I) Sulfates (SO) Insoluble Ionic Compounds Carbonates (CO3) Phosphates (PO) Hydroxides (OH-) 2+ Ag+, Hg+, Pb+ Hg+, Pb+, Sr+, Ba+ Group 1A cations,. NH Group 1A cations, NH Group 1A cations, NH, Sr+, Ba+
Step by Step Solution
3.45 Rating (148 Votes )
There are 3 Steps involved in it
a Write the table showing the ions and all of their possible combinations The tw... View full answer

Get step-by-step solutions from verified subject matter experts


