(0.10 mathrm{~mol}) of gas undergoes the process (1 ightarrow 2) shown in Figure P12.83. a. What...
Question:
\(0.10 \mathrm{~mol}\) of gas undergoes the process \(1 \rightarrow 2\) shown in Figure P12.83.
a. What are temperatures \(T_{1}\) and \(T_{2}\) ?
b. What type of process is this?
c. The gas undergoes constant-volume heating from point 2 until the pressure is restored to the value it had at point 1. What is the final temperature of the gas?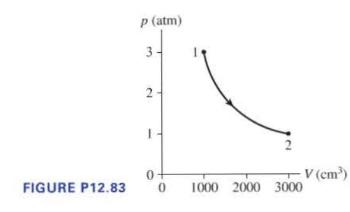
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

College Physics A Strategic Approach
ISBN: 9780321907240
3rd Edition
Authors: Randall D. Knight, Brian Jones, Stuart Field
Question Posted:





