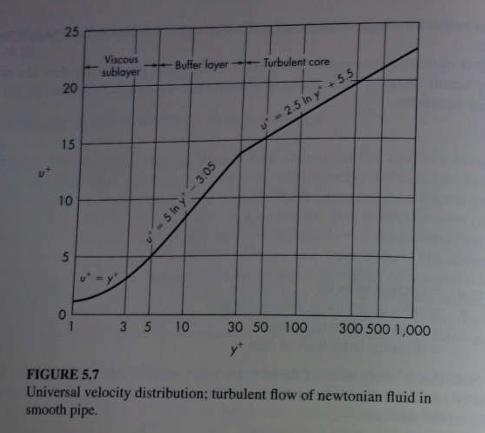
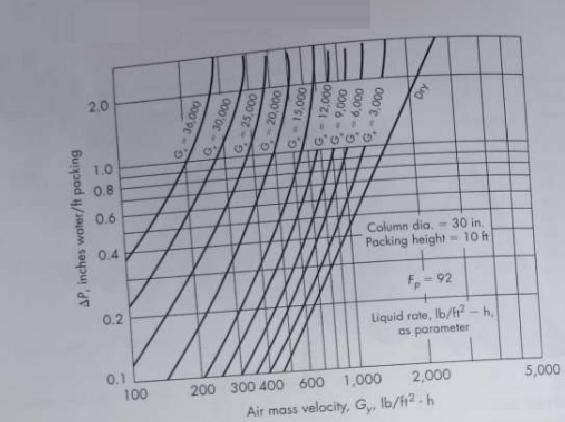
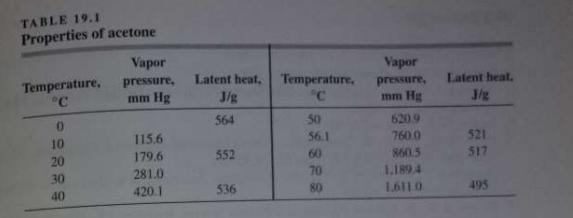
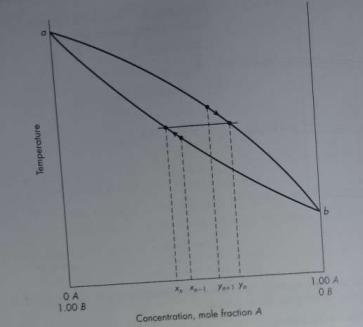
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


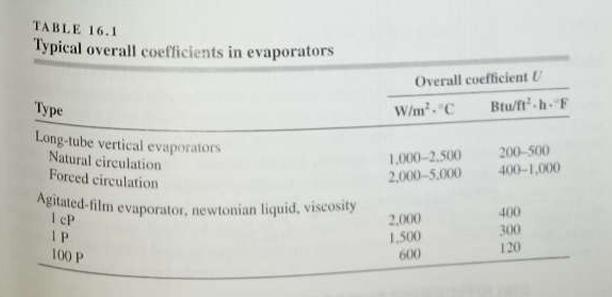
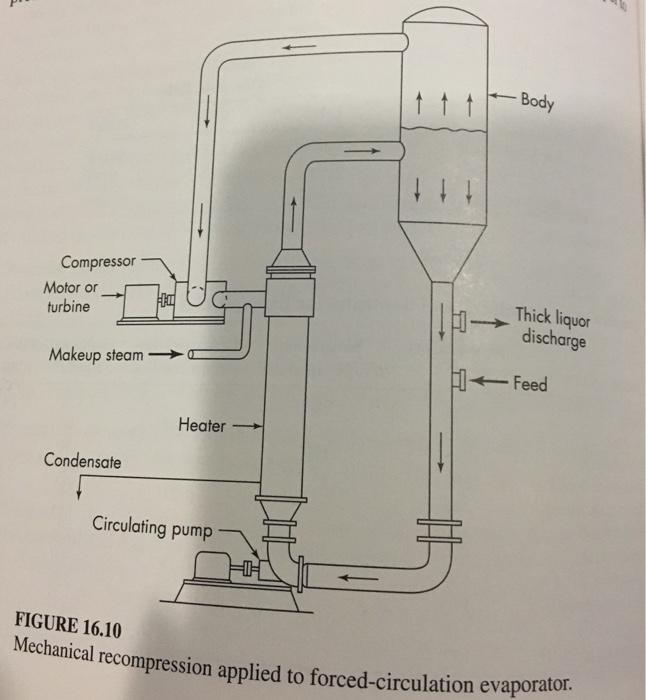
![DAR 0.00185873/21(MA+MB)/MAMB]/2 POD (17.28)](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1697/7/1/2/320653108c0bcc57123.png)