Consider a spherical gel bead containing a bio catalyst uniformly distributed within the gel. Within the gel
Question:
a. Define the system, and identify the source and the sink for the mass-transfer process with respect to reactant A. List three reasonable assumptions for this process. Then, using the €œshell balance€ approach, develop the differential material balance model for the process in terms of concentration profile cA. State all boundary conditions necessary to completely specify this differential equation.
b. The analytical solution for the concentration profile is given by
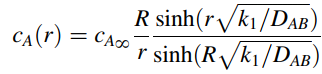
What is the total consumption rate of solute A by one single bead in units of µmol A per hour? The bead is 6.0 mm in diameter. The diffusion coefficient of solute A within the gel is 2 · l0-6 cm2/s, k1 is 0.019 s-1, and CAˆž is 0.02 µmole/cm3. Differentiate the relationship for cA(r) with respect to r, then estimate the flux NA at r = R.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster
Question Posted:





