A liquid mixture of 20% by mole benzene and the rest 2-propanol is in equilibrium with its
Question:
A liquid mixture of 20% by mole benzene and the rest 2-propanol is in equilibrium with its vapor at 69°C. Estimate the equilibrium pressure and the composition of the vapor phase. Assume the 1- parameter Margules equation holds. At this
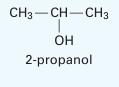
temperature, the vapor pressure of the benzene is 530 mm Hg and that of the 2-propanol is 434 mm Hg. Note that the system does form an azeotrope at this temperature whose composition is 61% by mole benzene (at a pressure of 685 mm Hg) (Storonkin and Morachevsky, 1956) If you make an assumption, please justify your assumption based on the results.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





