We noted in Figure 14-17 that the liquid and vapor curves taken together outline a lens-shaped region
Question:
We noted in Figure 14-17 that the liquid and vapor curves taken together outline a lens-shaped region when the normal boiling points of benzene-toluene solutions are plotted as a function of mole fraction of benzene. That is, unlike Figure 14-16, the liquid curve is not a straight line. Use data from Figure 14-17 and show by calculation that this should be the case.
Figure 14-17
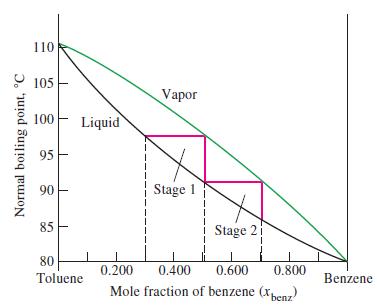
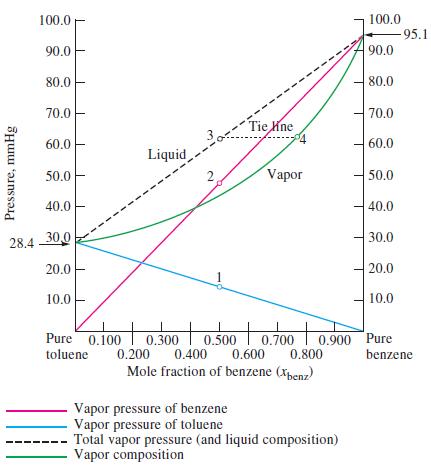
Figure 14-16
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





