With a minimum of calculation, determine how much less heat is produced per mol of C 8
Question:
With a minimum of calculation, determine how much less heat is produced per mol of C8H18(l) burned in reaction (21.30) than in reaction (21.29).
Reaction (21.30)
![]()
Reaction (21.29)
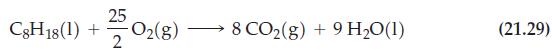
Transcribed Image Text:
C8H18(1) + 12 O₂(g) - 7 CO2(g) + CO(g) + 9H₂0(1) (21.30)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To determine how much less heat is produced per mol of C8H18l burned in reaction 2130 compared to re...View the full answer

Answered By

Michael Mulupi
I am honest,hardworking, and determined writer
4.70+
72+ Reviews
157+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
a fashion-boutique chain store, with 13 units, in Washington, D. C. area is planning a big sale in its suburban warehouse on the three-day Labor Day weekend (Saturday through Monday.) On sale will be...
-
Plot the six risks on a probability/impact matrix using the project impact matrix 2023 template. Write one to two sentences stating the rationale for how you determined the quadrant placement for...
-
Why are supplies not considered inventory? What type of account is Supplies on Hand?
-
Penguin Corporation acquired 80 percent of the outstanding voting stock of Snow Company on January 1, 2010, for $420,000 in cash and other consideration. At the acquisition date, Penguin assessed...
-
Given the background reading, list three verbal deception and three non-verbal deception cues that you believe that Aldrich Ames might exhibit and describe why
-
Sarmento Tax Services prepares tax returns for senior citizens. The standard in terms of (direct labor) time spent on each return is 2.0 hours. The direct labor standard wage rate at the firm is $...
-
Suppose your yearly demand for razors is Q = 32 - 4P. There is a subscription service that charges $2.00 per razor plus an annual membership fee. What is the most that you would be willing to pay for...
-
Suggest a reason why the hydrated beryllium ion is [Be(H 2 O) 4 ] 2+ , whereas the hydrated magnesium ion is [Mg(H 2 O) 6 ] 2+ .
-
Arrange the following compounds in the expected order of increasing solubility in water, and give the basis for your arrangement: Li2CO 3 , Na 2 CO 3 , MgCO 3 .
-
Using data from Appendix A, compute the Q value for the reaction (a) 12 C(a, p) 15 N, and (b) 16 O(p, d) 17 O.
-
Go to http://www.federalreserve.gov/releases/h6/hist/ and find the historical report of M1 and M2 by clicking on the Data Download Program. Compute the growth rate of each aggregate over each of the...
-
Consider a representative agent economy where the representative agent's utility function is given by \(\mathbf{u}(x)=\log (x)\) and the aggregate endowment process is \(e=\left\{e_{0}, e\left(A_{t}...
-
Giving justifications for all your decisions, advise Frank and his staff at Hill Street Motorist Shop (case study 8) on all of the aspects of search engine optimization covered in this chapter....
-
Each student should obtain the statement of cash flows from the annual report of a favorite company. Annual reports are usually downloadable from the website of a public company, or are otherwise...
-
When the competition is stiff, the product, service, and price may not be the deciding factor. What the buyer believes about the competitor may be controlling. The following are statements made by...
-
Marcus is the vice president of human resources for Griffin Industries. He spent one week testifying before Congress on the impact health care legislation will have on small business. His trip cost...
-
Akramin just graduated with a Master of Engineering in Manufacturing Engineering and landed a new job in Melaka with a starting salary of RM 4,000 per month. There are a number of things that he...
-
Using the data in question 13, how would Diana report the data if the investment were long-term and the securities were classified as available-for-sale?
-
Roso Companys investments in available-for-sale securities at December 31 show total cost of $202,000 and total fair value of $210,000. Prepare the adjusting entry.
-
Using the data in question 15, prepare the adjusting entry assuming the securities are classified as trading securities.
-
How do theories of social identity and group dynamics inform our understanding of intractable conflicts, exploring the role of collective identities, group cohesion, and intergroup relations in...
-
Assume that your calculation for the total difference in performance for the replacements is $121,400, discuss the implication of this value. Then calculate the financial impact of this turnover and...
-
Select a criminal justice program that would be subject to evaluation. It can be from any state, local, or federal organization. Describe the criminal justice program you selected. Identify the...

Study smarter with the SolutionInn App


