A mass m of liquid water at temperature T 1 is mixed adiabatically and isobarically with an
Question:
A mass m of liquid water at temperature T1 is mixed adiabatically and isobarically with an equal mass of liquid water at temperature T2. Assuming constant CP, show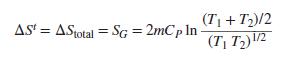 and prove that this is positive. What would be the result if the masses of the water were different, say, m1 and m2?
and prove that this is positive. What would be the result if the masses of the water were different, say, m1 and m2?
Transcribed Image Text:
(T1 + T2)/2 AS' = AStotal = SG = 2mCp In %3D (T| T2)\/2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
If a mass m of liquid water at temperature T1 is mixed adiabatically and isobarically wit...View the full answer

Answered By

Dulal Roy
As a tutor, I have gained extensive hands-on experience working with students one-on-one and in small group settings. I have developed the ability to effectively assess my students' strengths and weaknesses, and to customize my teaching approach to meet their individual needs.
I am proficient at breaking down complex concepts into simpler, more digestible pieces, and at using a variety of teaching methods (such as visual aids, examples, and interactive exercises) to engage my students and help them understand and retain the material.
I have also gained a lot of experience in providing feedback and guidance to my students, helping them to develop their problem-solving skills and to become more independent learners. Overall, my hands-on experience as a tutor has given me a deep understanding of how to effectively support and encourage students in their learning journey.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
What would be the result if the only insider on a corporations board were the CEO?
-
An ice tray contains 500 g of liquid water at 0C. Calculate the change in entropy of the water as it freezes slowly and completely at 0C.
-
A container filled with 45 kg of liquid water at 95°C is placed in a 90-m3 room that is initially at 12°C. Thermal equilibrium is established after a while as a result of heat transfer...
-
During the software design phase, software engineers define details about the product construction, behavior, components, and interfaces. Explain how you can use the Unified Modeling Language (UML)...
-
List three of the most common mistakes made during an interview.
-
The following costs are labeled fixed or variable according to a typical designation in accounting. Under which circumstances would any of these costs behave in a manner opposite to that listed? a....
-
Identify three functions for an ATM system.
-
Tom Loper is an accounting major at a midwestern state university located approximately 60 miles from a major city. Many of the students attending the university are from the metropolitan area and...
-
Fairfield Company's raw materials inventory transactions for the most recent month are summarized here: Note: Assume, purchase of raw materials is on account. Beginning raw materials Purchases of raw...
-
Three friends, Optimist, Realist, and Pessimist, go to a casino. They decide to play a gambling game for which they do not know the probability p of winning. Motivated by an exciting lecture on...
-
For an ideal gas prove that: AS c dT V + In, Vo R To R T
-
Reversible adiabatic processes are isentropic. Are isentropic processes necessarily reversible and adiabatic? If so, explain why; if not, give an illustrative example.
-
During 2012 volunteer pinstripers donated their services to General Hospital at no cost. The staff at General Hospital was in control of the pinstripers' duties. If regular employees had provided the...
-
Develop a data type ResizingArrayQueueOfStrings that implements a queue with a fixed-length array in such a way that all operations take constant time. Then, extend your implementation to use a...
-
What would be the effect of using a queue instead of a stack when forming the shortest path in pathTo()?
-
When using generics, what happens if I omit the type argument in either the declaration or the constructor call? \begin{tabular}{llll} Stack stack & \(=\) new Stack ()\(;\) & & // unsafe \\ Stack &...
-
Perform computational experiments to verify that the average path length in a ring graph on \(V\) vertices is \(\sim 1 / 4 V\). Then, repeat these experiments, but add one random edge to the ring...
-
Develop a class Stack0fInts that uses a linked-list representation (but no generics) to implement a stack of integers. Write a client that compares the performance of your implementation with Stack...
-
Show that if W R3 is a subspace containing the vectors (1,2, - l)T, (2,0. l)T, (0,-1,3)T, then W = R3.
-
You purchase a bond with a coupon rate of 6.7 percent, a par value $1,000, and a clean price of $905. Assume a par value of $1,000. If the next semiannual coupon payment is due in two months, what is...
-
The temperature dependence of the second virial coefficient B is shown for nitrogen in Fig. 3.8. Qualitatively, the shape of B(T) is the same for all gases; quantitatively, the temperature for which...
-
Starting with Eq. (6.9), show that isotherms in the vapor region of a Mollier (HS) diagram have slopes and curvatures given by: Here, is volume expansivity. If the vapor is described by the two-term...
-
A well-insulated tank of 50 m 3 volume initially contains 16,000 kg of water distributed between liquid and vapor phases at 25C. Saturated steam at 1500 kPa is admitted to the tank until the pressure...
-
Information that does not have an adjustment reason code to route payments into the correct bank accounts to relay about patient benefit coverage to indicate the amount being paid and the date of...
-
Superior Company provided the following data for the year ended December 31 (all raw materials are used in production as direct materials): Selling expenses Purchases of raw materials Direct labor...
-
The art of negotiation is especially important when working in an international business setting. Negotiating a successful contract is a highly valuable skill that is to be attained by working on...
Communication And Journalism Notebook Journal Composition 1st Edition - ISBN: 1722487852 - Free Book

Study smarter with the SolutionInn App


