PVT data may be taken by the following procedure: A mass m of a substance of molar
Question:
PVT data may be taken by the following procedure: A mass m of a substance of molar mass ℳ is introduced into a thermostated vessel of known total volume Vt. The system is allowed to equilibrate, and the temperature T and pressure P are measured.
(a) Approximately what percentage errors are allowable in the measured variables (m, ℳ, Vt, T, and P) if the maximum allowable error in the calculated compressibility factor Z is ±1%?
(b) Approximately what percentage errors are allowable in the measured variables if the maximum allowable error in calculated values of the second virial coefficient B is ±1%? Assume that Z ≃ 0.9 and that values of B are calculated by Eq. (3.37).
Eq. (3.37)
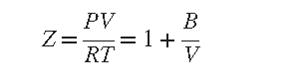
Step by Step Answer:

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart





