Question: Predict whether the ground state or the first excited state of CH 2 should have the larger bond angle on the basis of the Walsh
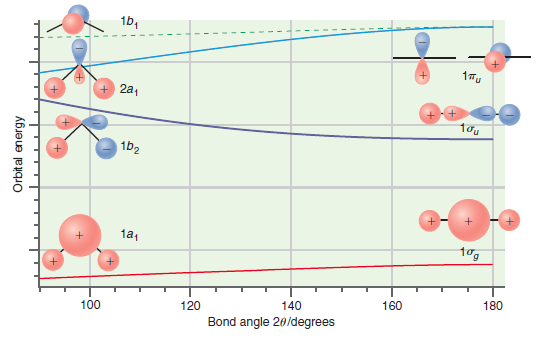
1b, 17u 2a, 1b2 1a, tog 180 160 140 120 100 Bond angle 20/degrees Orbit al energy
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
CH 2 has six valence electrons In the ground state the HOMO is the 2a ... View full answer

Get step-by-step solutions from verified subject matter experts


