Question: A chemist mixes solid AgCl. CuC12, and MgC12 in enough water to give a final volume of 50.0 mL. (a) With ions shown as spheres
A chemist mixes solid AgCl. CuC12, and MgC12 in enough water to give a final volume of 50.0 mL. (a) With ions shown as spheres and solvent molecules omitted for clarity, which scene best represents the resulting mixture? 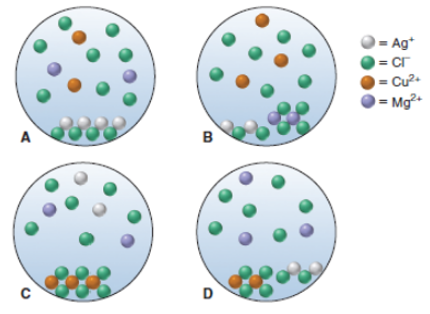
(b) If each sphere represents 5.0 ? 10 -3 mol of ions, what is the total concentration of dissolved (separated) ions?
(c) What is the total mass of solid?
A Bos B D of Ag+ =cr = Cu+ = Mg+
Step by Step Solution
3.28 Rating (151 Votes )
There are 3 Steps involved in it
To address this problem well consider the solubility of each compound in water a Identify the scene ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
60946b216f29d_24825.pdf
180 KBs PDF File
60946b216f29d_24825.docx
120 KBs Word File


