Question: 7. Consider the chromate/dichromate ion equilibrium: + 2 Cro 2- (aq) + 2 H+ (aq) Cr,0,2- (aq) + H20 0 yellow orange a) What color
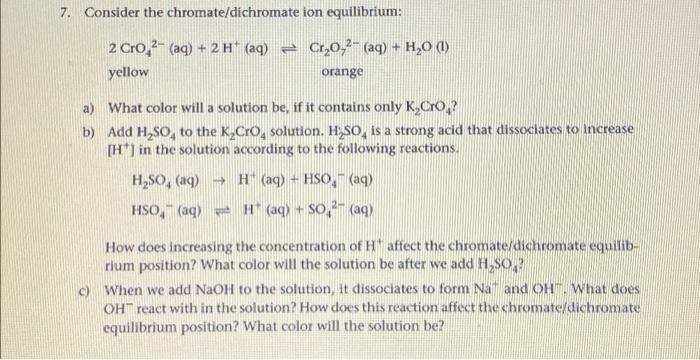
7. Consider the chromate/dichromate ion equilibrium: + 2 Cro 2- (aq) + 2 H+ (aq) Cr,0,2- (aq) + H20 0 yellow orange a) What color will a solution be, if it contains only K,Cros? b) Add H So, to the K,Cro solution. H,SO, is a strong acid that dissociates to increase [H] in the solution according to the following reactions H,SO, (aq) H(aq) + HSO - (aq) HSO, (aq) = H (aq) + s0,3- (aq) How does increasing the concentration of Haffect the chromate/dichromate equilib- rium position? What color will the solution be after we add H SO c) When we add NaOH to the solution, it dissociates to form Na and Ohm. What does OH react with in the solution? How does this reaction affect the chromate/dichromate equilibrium position? What color will the solution be
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


