Question: A 100 mol mixture containing 62% n-pentane and 38 mol n-heptane is distilled under different conditions at 103.3 kPa to obtain 45 moles. What is
A 100 mol mixture containing 62% n-pentane and 38 mol n-heptane is distilled under different conditions at 103.3 kPa to obtain 45 moles. What is the average composition of the total distilled vapor and the remaining liquid?
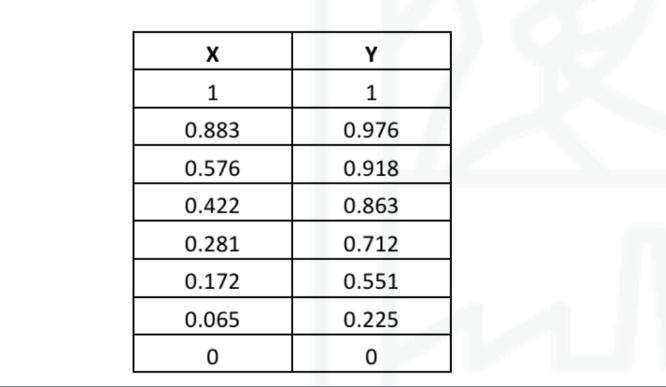
X Y 1 1 0.883 0.976 0.576 0.918 0.422 0.863 0.281 0.712 0.172 0.551 0.065 0.225 0 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


