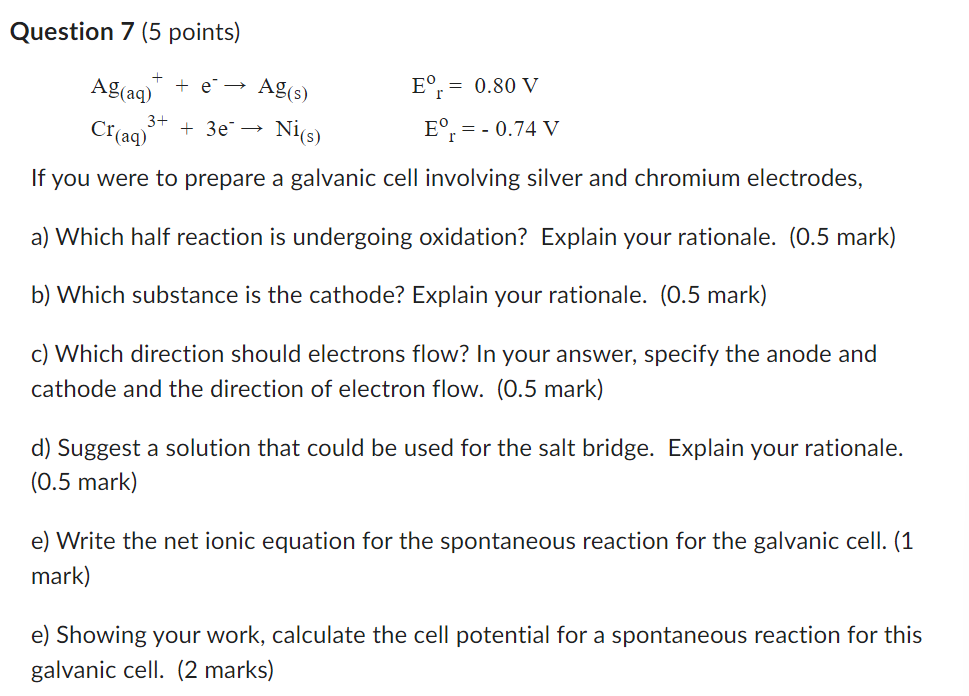
Question: Ag ( aq ) + + e - - > Ag ( s ) Eor = 0 . 8 0 V Cr ( aq )
Agaq e Ags Eor V
Craqe Nis Eor V
If you were to prepare a galvanic cell involving silver and chromium electrodes,
a Which half reaction is undergoing oxidation? Explain your rationale. mark
b Which substance is the cathode? Explain your rationale. mark
c Which direction should electrons flow? In your answer, specify the anode and cathode and the direction of electron flow. mark
d Suggest a solution that could be used for the salt bridge. Explain your rationale. mark
e Write the net ionic equation for the spontaneous reaction for the galvanic cell. markQuestion points
If you were to prepare a galvanic cell involving silver and chromium electrodes,
a Which half reaction is undergoing oxidation? Explain your rationale. mark
b Which substance is the cathode? Explain your rationale. mark
c Which direction should electrons flow? In your answer, specify the anode and
cathode and the direction of electron flow. mark
d Suggest a solution that could be used for the salt bridge. Explain your rationale.
mark
e Write the net ionic equation for the spontaneous reaction for the galvanic cell.
mark
e Showing your work, calculate the cell potential for a spontaneous reaction for this
galvanic cell. marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


