Question: 1) Consider barium sulfate crystals in saturated aqueous solution with interfacial energy of 120 mJ/m? and Gibbs free energy barrier of 5.02x1019 J a.
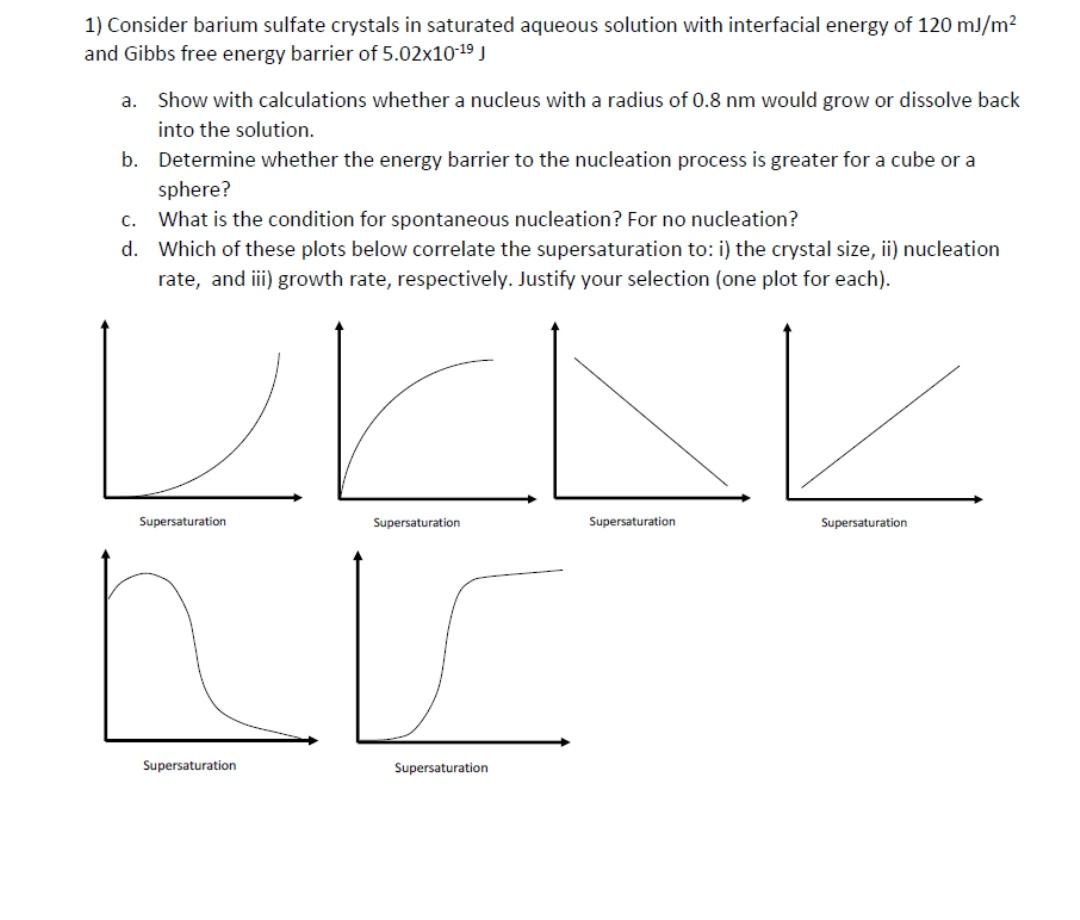
1) Consider barium sulfate crystals in saturated aqueous solution with interfacial energy of 120 mJ/m? and Gibbs free energy barrier of 5.02x1019 J a. Show with calculations whether a nucleus with a radius of 0.8 nm would grow or dissolve back into the solution. b. Determine whether the energy barrier to the nucleation process is greater for a cube or a sphere? c. What is the condition for spontaneous nucleation? For no nucleation? d. Which of these plots below correlate the supersaturation to: i) the crystal size, ii) nucleation rate, and i) growth rate, respectively. Justify your selection (one plot for each). Supersaturation Supersaturation Supersaturation Supersaturation Supersaturation Supersaturation
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


