Question: Copper(I) ions in aqueous solution react with NH, (aq) according to Cu*(aq) + 2 NH,(aq) Cu(NH, )* (aq) Kf = 6.3 x 1010 Calculate
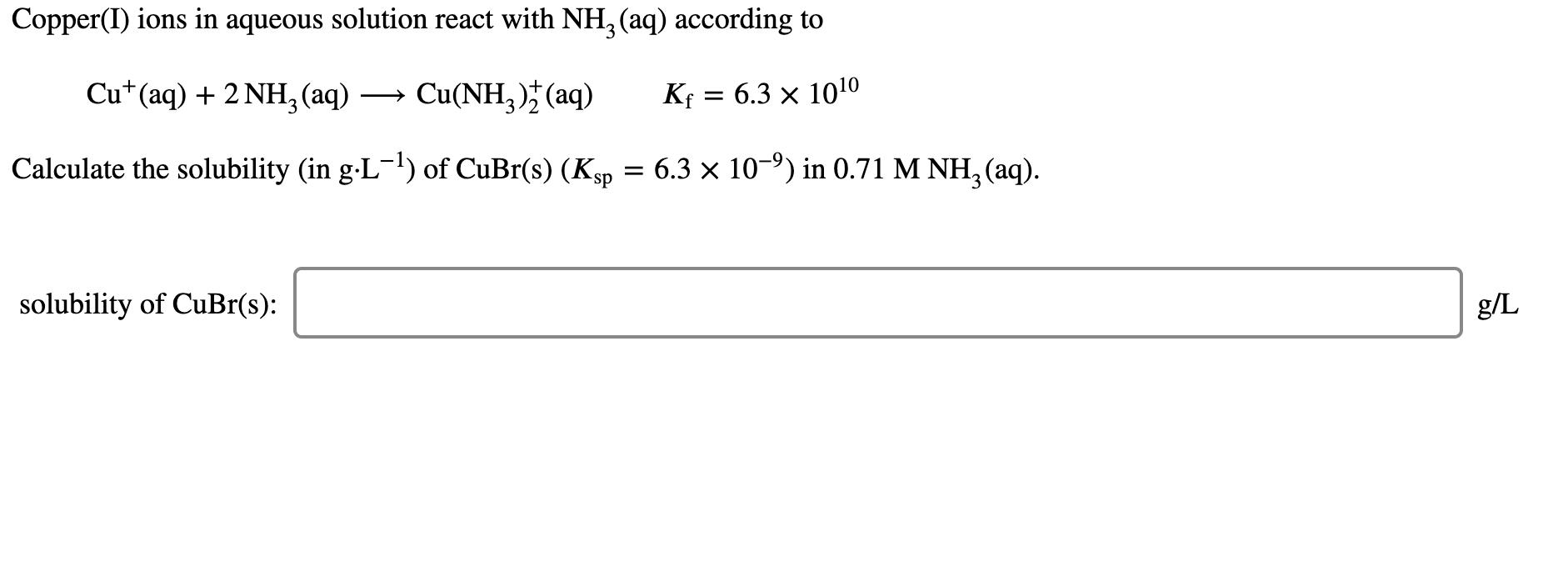
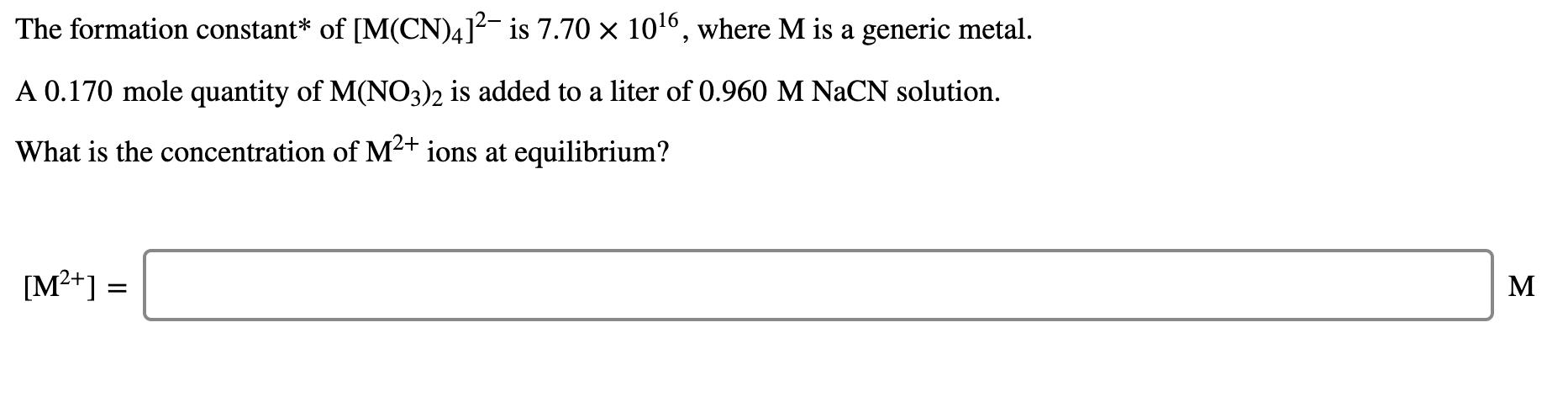
Copper(I) ions in aqueous solution react with NH, (aq) according to Cu*(aq) + 2 NH,(aq) Cu(NH, )* (aq) Kf = 6.3 x 1010 Calculate the solubility (in g-L') of CuBr(s) (Ksp = 6.3 x 10-9) in 0.71 M NH, (aq). solubility of CuBr(s): g/L The formation constant* of [M(CN)4]?- is 7.70 1016, where M is a generic metal. A 0.170 mole quantity of M(N03)2 is added to a liter of 0.960 M NACN solution. What is the concentration of M+ ions at equilibrium? [M+] = M
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


