Question: For a first-order reaction, the initial reactant concentration is 0.784M. After 14.1min, the concentration is 0.325M. What is k in min
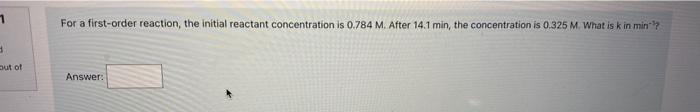
For a first-order reaction, the initial reactant concentration is 0.784M. After 14.1min, the concentration is 0.325M. What is k in min
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


