Question: please do all or most For a first-order reaction, the initial reactant concentration is 0.727M. After 86.6 ming the concentration is 0.422M. What is k
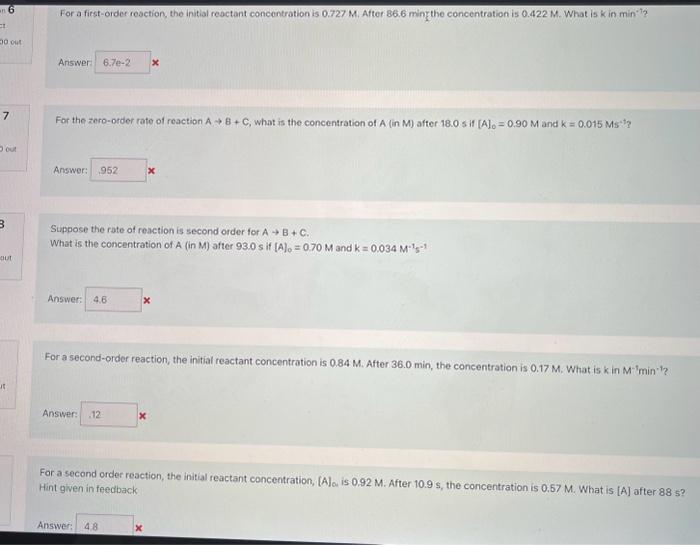
For a first-order reaction, the initial reactant concentration is 0.727M. After 86.6 ming the concentration is 0.422M. What is k in min - T? For the zero-order rate of reaction AB+C, what is the concentration of A (in M ) after 18.0 s if [A]0=0.90M and K=0.015Ms1 ? Suppose the rate of reaction is second order for AB+C. What is the concentration of A (in M ) after 93.0 s if [A]0=0.70M and K=0.034M1s1 For a second-order reaction, the initial reactant concentration is 0.84M. After 36.0 min, the concentration is 0.17MWhat, is kinM1min1 ? For a second order reaction, the initial reactant concentration, [A]0, is 0.92M. After 10.9s, the concentration is 0.67M. What is [A] after 885 ? Hint given in feedback Answer: x
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


