Question: please solve both!! For a first-order reaction, the initial reactant concentration is 0.727M. After 86.6min, the concentration is 0.422M. What is k in min1 ?

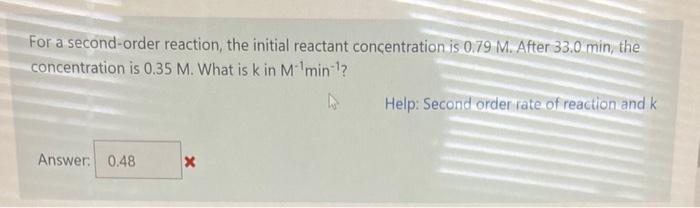
For a first-order reaction, the initial reactant concentration is 0.727M. After 86.6min, the concentration is 0.422M. What is k in min1 ? Help: First order rate of reaction Answer: For a second-order reaction, the initial reactant concentration is 0.79M. After 33.0min, the concentration is 0.35M. What is k in M1min1 ? Help: Second order rate of reaction and k
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


