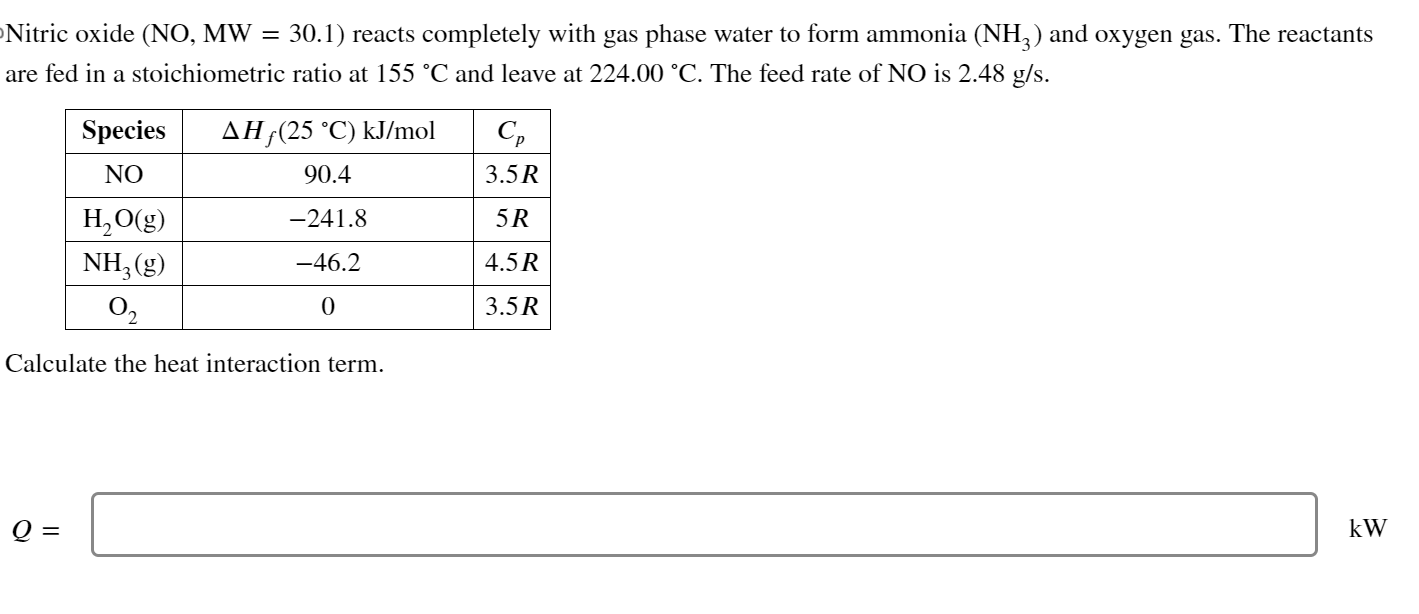
Question: Nitric oxide ( N O , M W = 3 0 . 1 ) reacts completely with gas phase water to form ammonia ( N
Nitric oxide reacts completely with gas phase water to form ammonia and oxygen gas. The reactants
are fed in a stoichiometric ratio at and leave at The feed rate of is
Calculate the heat interaction term.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


