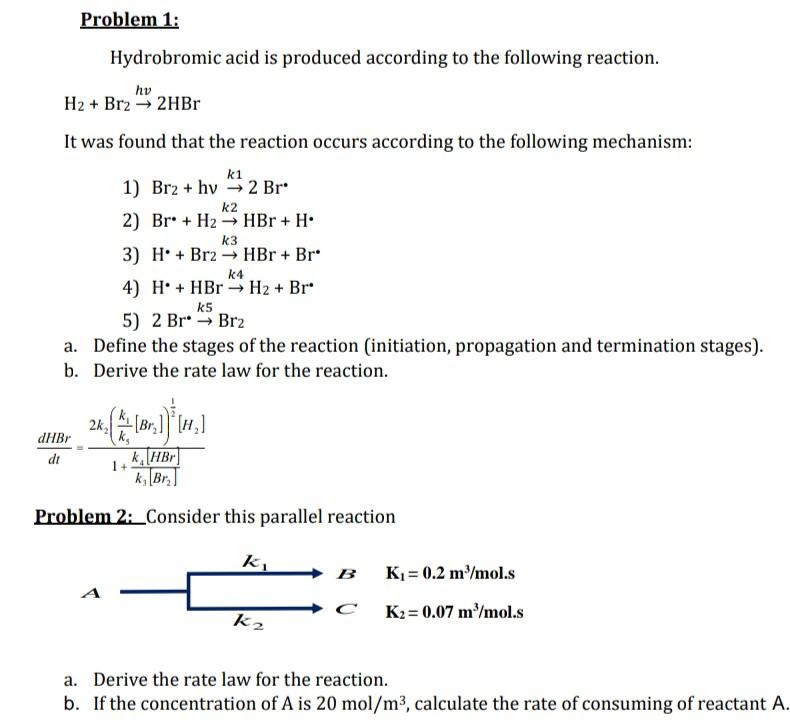
Question: okay answer one of them in a computerised form please Hydrobromic acid is produced according to the following reaction. H2+Br2hv2HBr It was found that the
okay answer one of them in a computerised form please
Hydrobromic acid is produced according to the following reaction. H2+Br2hv2HBr It was found that the reaction occurs according to the following mechanism: 1) Br2+hvk12Br 2) Br+H2k2HBr+H 3) H+Br2k3HBr+Br 4) H+HBrH2+Br 5) 2Brk5Br2 a. Define the stages of the reaction (initiation, propagation and termination stages). b. Derive the rate law for the reaction. dtdHBr=1+k3[Br2]k4[HBr]2k2(k5k1[Br2])21[H2] Problem 2: Consider this parallel reaction a. Derive the rate law for the reaction. b. If the concentration of A is 20mol/m3, calculate the rate of consuming of reactant A. Hydrobromic acid is produced according to the following reaction. H2+Br2hv2HBr It was found that the reaction occurs according to the following mechanism: 1) Br2+hvk12Br 2) Br+H2k2HBr+H 3) H+Br2k3HBr+Br 4) H+HBrH2+Br 5) 2Brk5Br2 a. Define the stages of the reaction (initiation, propagation and termination stages). b. Derive the rate law for the reaction. dtdHBr=1+k3[Br2]k4[HBr]2k2(k5k1[Br2])21[H2] Problem 2: Consider this parallel reaction a. Derive the rate law for the reaction. b. If the concentration of A is 20mol/m3, calculate the rate of consuming of reactant A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


