Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #3 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #3!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
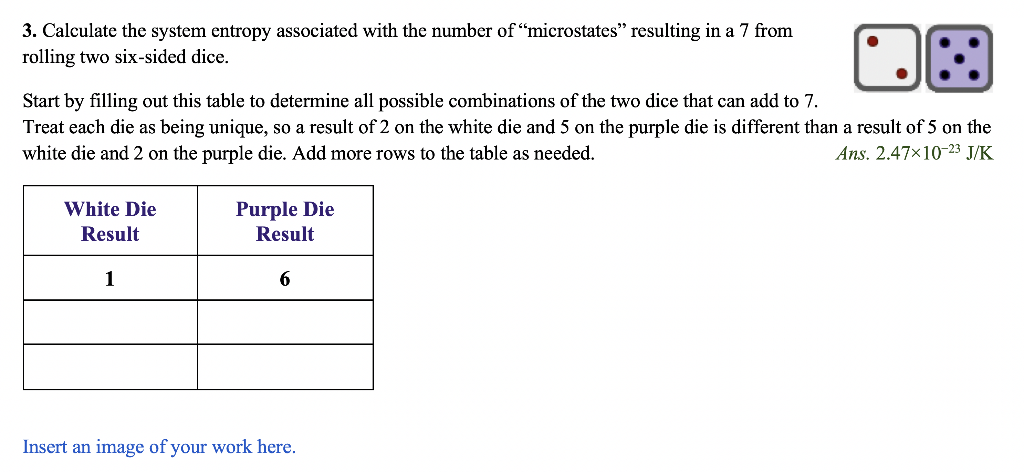
Part 2 - Boltzmann's Equation for Entropy Ludwig Boltzmann developed an equation to calculate the entropy of a system: S=klnW where S is entropy, W is the number of microstates (possible ways to arrange the system), and k is the Boltzmann constant (1.381023J/K) 3. Calculate the system entropy associated with the number of "microstates" resulting in a 7 from rolling two six-sided dice. Start by filling out this table to determine all possible combinations of the two dice that can add to 7. Treat each die as being unique, so a result of 2 on the white die and 5 on the purple die is different than a result of 5 on the white die and 2 on the purple die. Add more rows to the table as needed. Ans. 2.471023J/K Insert an image of your work here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


