Question: Please, if you know the answer, let me know! The following compounds are only slightly soluble in water. One of these compounds is very soluble
Please, if you know the answer, let me know!
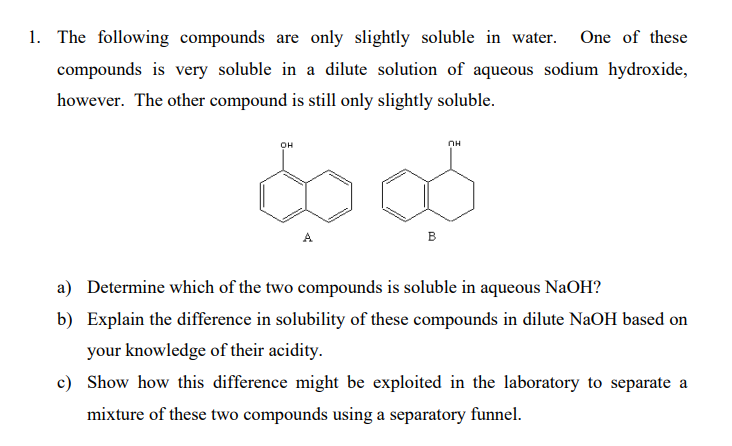
The following compounds are only slightly soluble in water. One of these compounds is very soluble in a dilute solution of aqueous sodium hydroxide, however. The other compound is still only slightly soluble. a) Determine which of the two compounds is soluble in aqueous NaOH ? b) Explain the difference in solubility of these compounds in dilute NaOH based on your knowledge of their acidity. c) Show how this difference might be exploited in the laboratory to separate a mixture of these two compounds using a separatory funnel
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


