Question: Problem 1 ( 5 points ) The elementary reversible liquid phase reaction: A + Bharr 2 C occurs in an isothermal mixed flow reactor operated
Problem points
The elementary reversible liquid phase reaction: Bharr occurs in an isothermal mixed flow reactor operated at The feed is equal molar in A and with the total flow rate of mol and the initial concentration of A equal to moleliter Calculate the reactor volume needed to achieve of the equilibrium conversion.
Additional information for the problem:
litermole:min
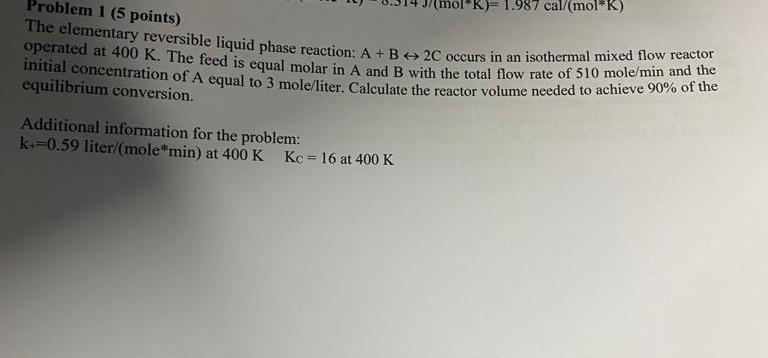
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


