Question: Use the References to access important values if needed for this question. A student is running an experiment in which 31.8 grams of Na2CrO4 is
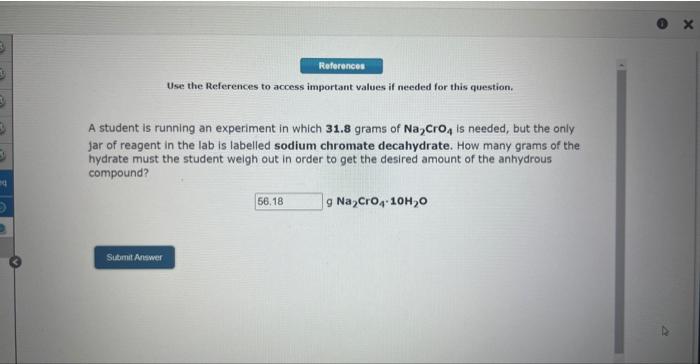
Use the References to access important values if needed for this question. A student is running an experiment in which 31.8 grams of Na2CrO4 is needed, but the only jar of reagent in the lab is labelled sodium chromate decahydrate. How many grams of the hydrate must the student weigh out in order to get the desired amount of the anhydrous compound
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


