In the presence of nitric acid, UO 2+ undergoes a redox process. It is converted to UO
Question:
In the presence of nitric acid, UO2+ undergoes a redox process. It is converted to UO22+ and nitric oxide (NO) gas is produced according to the following unbalanced equation:
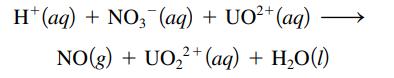
If 2.55 × 102 mL NO(g) is isolated at 29οC and 1.5 atm, what amount (moles) of UO2+ was used in the reaction?
Transcribed Image Text:
H+ (aq) + NO3(aq) + UO²+ (aq) NO(g) + UO₂²+ (aq) + H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Amount moles of ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
If 2-methylpropane is brominated at 125C in the presence of light, what percent of the product will be 2-bromo-2-methylpropane? Compare your answer with the percent given in Problem 4 for...
-
In the presence of the enzyme aconitase, the double bond of aconitic acid undergoes hydration. The reaction is reversible, and the following equilibrium is established:
-
At 500 K in the presence of a copper surface, ethanol decomposes according to the equation C2H5OH(g) CH3CHO(g) + H2(g) The pressure of C2H5OH was measured as a function of time, and the following...
-
Why do we need Normalization? How many forms of Normalization exist? What is the minimum normal form required? Describe each normal form List Normalization conversion process
-
In Shelanu v. Three Print, the Ontario Court of Appeal held that franchisors owe a duty of good faith to franchisees. What factors led the court to this conclusion? In what other contractual...
-
Write an HDL module for a hexadecimal seven-segment display decoder. The decoder should handle the digits A, B, C, D, E, and F as well as 09.
-
A rectangular wooden column has the cross section shown. If \(a=3\) in. and the column is subjected to an axial force of \(P=15\) kip, determine the maximum length the column can have to safely...
-
Capital Project Transactions. In 2011, Falts City began work to improve certain streets to be financed by a bond issue and supplemented by a federal grant. Estimated total cost of the project was...
-
Use the information below to answer the two questions that follow. Atwood Corporation uses the weighted-average method in its process-costing system. Operating data for the first department for May...
-
The Katash Company is a leader in the poultry market. It produces, sells and markets fresh and ice packed commodity chicken and frozen products known for their value and healthful qualities. Katash's...
-
We state that the ideal gas law tends to hold best at low pressures and high temperatures. Show how the van der Waals equation simplifies to the ideal gas law under these conditions.
-
A large flask with a volume of 936 mL is evacuated and found to have a mass of 134.66 g. It is then filled to a pressure of 0.967 atm at 31 C with a gas of unknown molar mass and then reweighed to...
-
Analyzing the Performance of an Import/Export Department William Johnston manages the import/export department of Bush Specialty Products. Because of the complexities of foreign currency transactions...
-
How do neuroeconomic principles shed light on the neural mechanisms underlying decision-making under risk and uncertainty?
-
Kingbird Corporation operates in an industry that has a high rate of bad debts. Before any year-end adjustments, the balance in Kingbird's Accounts Receivable account was $581,000 and Allowance for...
-
Jonathan, Stuart, and Anna created BBJ, a website design partnership on January 1, 2023. Jonathan invested $2,000 cash and a computer worth $3,000. Stuart invested $6,000 cash and Anna invested...
-
3. Given the matrix S= = [ = 11 36 -3 -10 6] a) Compute for its eigenvalues and their corresponding eigenvectors. (Select eigenvectors with the smallest possible whole number entries). [6pts] b) Let...
-
A convertible bond has a conversion rate of 2.5 shares of stock per $1000 face value bond. Today, the stock currently trades at $385 per share. Also, the convertible bond is trading at 97.5 percent...
-
Suppose that A = SAS-1 where is a diagonal matrix with diagonal elements 1, 2, . . . , n. (a) Show that ASi = iSi i = 1,..., n. (b) Show that if x = a1S1 + a2s2 + ns2 +.....+ then Akx = 1k1s1 +...
-
Kenneth Hubbard has prepared the following list of statements about managerial accounting and financial accounting. 1. Financial accounting focuses on providing information to internal users. 2....
-
A compound with molecular formula C 10 H 10 O 4 produces a 1 H NMR spectrum that exhibits only two signals, both singlets. One signal appears at 3.9 ppm with a relative integration value of 79. The...
-
For each of the following compounds, predict the number of signals and location of each signal in a 13 C NMR spectrum: (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) H.
-
Compare the following two constitutional isomers. The 13 C NMR spectrum of the first compound exhibits five signals, while the second compound exhibits six signals. Explain. .
-
The following account balances are taken from the ledger of Maxwell Limited on 31 December 2018, the end of its fiscal year: Maxwell Limited Trial Balance As on 31 December 2018 S'm S'm Investment...
-
A researcher wishes to assess whether vitamin C is effective in the treatment of colds. To evaluate this hypothesis, the researcher decides to conduct a 2-year experimental study. The researcher...
-
Cullumber Co. acquired 25% of the 520,000 shares of outstanding common stock of Oriole Inc. on December 31, 2025. The purchase price was $3,913,000. Oriole declared and paid $1.20 per share cash...

Study smarter with the SolutionInn App


