The irreversible elementary gas-phase reaction A+BC+D is carried out isothermally at 305 K in a packed-bed reactor
Question:
The irreversible elementary gas-phase reaction A+B→C+D is carried out isothermally at 305 K in a packed-bed reactor with 100 kg of catalyst.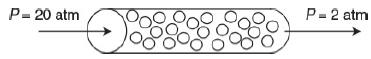
The entering pressure was 20 atm and the exit pressure is 2 atm. The feed is equal molar in A and B and the flow is in the turbulent flow regime, with FA0 = 10 mol/min and CA0 = 0.4 mol/dm3. Currently 80% conversion is achieved. What would be the conversion if the catalyst particle size were doubled and everything else remained the same?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





