Question: Use data from Table 23.8 to determine E for the reduction of Hg 2+ to Hg 2 2+ in aqueous solution. Table 23.8 TABLE 23.8
Use data from Table 23.8 to determine E° for the reduction of Hg2+ to Hg22+ in aqueous solution.
Table 23.8
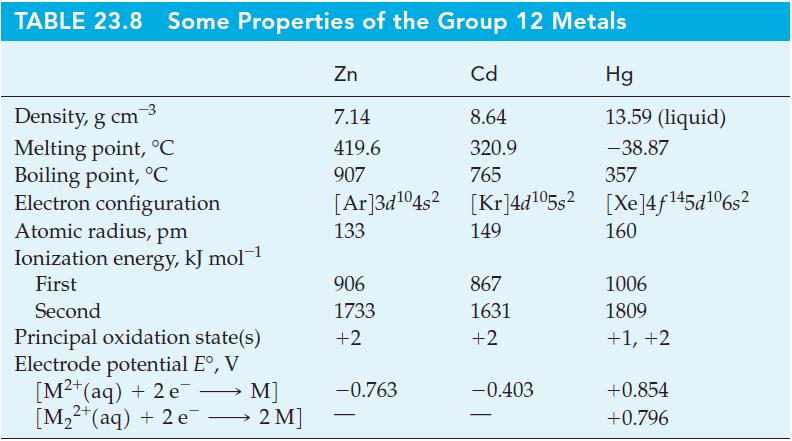
TABLE 23.8 Some Properties of the Group 12 Metals Density, g cm Melting point, C Boiling point, C Electron configuration Atomic radius, pm Ionization energy, kJ mol- First Second Principal oxidation state(s) Electrode potential E, V [M+ (aq) + 2 e [M+ (aq) + 2 e - M] 2 M] Zn 7.14 419.6 907 [Ar]3d104s 133 906 1733 +2 -0.763 Cd 8.64 320.9 765 [Kr]4d05s [kr]4d05s2 [Xe]4f145d06s 149 867 1631 +2 Hg 13.59 (liquid) -38.87 357 -0.403 160 1006 1809 +1, +2 +0.854 +0.796
Step by Step Solution
3.34 Rating (154 Votes )
There are 3 Steps involved in it
To determine the standard potential E for the reduction of Hg... View full answer

Get step-by-step solutions from verified subject matter experts


