Question: Comment on the reduction potential data in Table 19.1. Data from Table 19.1. Reduction half-equation Ca+ (aq) + 2e Ca(s) Ti+ (aq) + 2e Ti(s)
Comment on the reduction potential data in Table 19.1.
Data from Table 19.1.
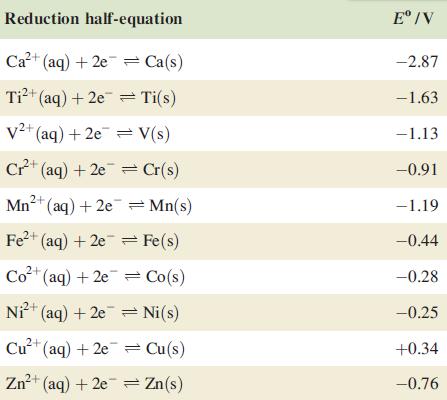
Reduction half-equation Ca+ (aq) + 2e Ca(s) Ti+ (aq) + 2e Ti(s) V+ (aq) +2e= V(s) Cr+ (aq) +2e=Cr(s) Mn+ (aq) + 2e = Mn(s) 2+ Fe+ (aq) +2e = Fe(s) Co+ (aq) + 2e Co(s) Ni+ (aq) +2e=Ni(s) Cu+ (aq) + 2e = Cu(s) Zn+ (aq) + 2e = Zn(s) E/V -2.87 -1.63 -1.13 -0.91 -1.19 -0.44 -0.28 -0.25 +0.34 -0.76
Step by Step Solution
3.52 Rating (155 Votes )
There are 3 Steps involved in it
The reduction potential data provided in Table 191 shows the standard reduction potentials E for various redox reactions Reduction potential is a meas... View full answer

Get step-by-step solutions from verified subject matter experts


