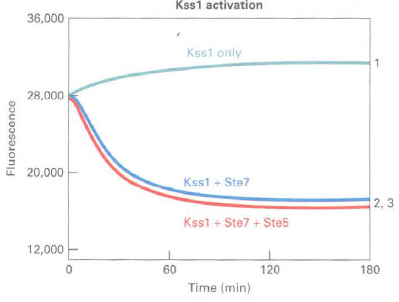
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()



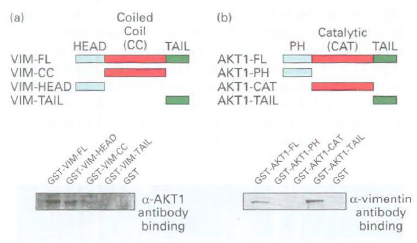
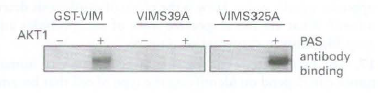
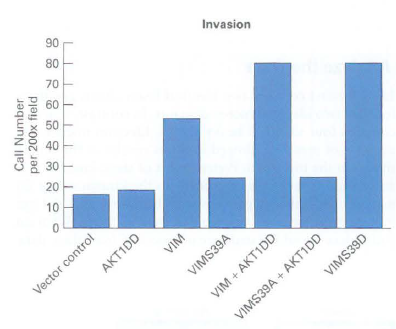
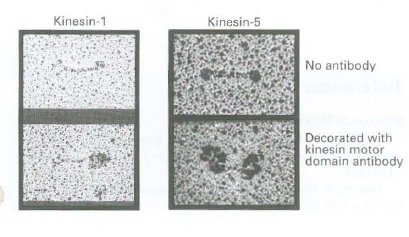
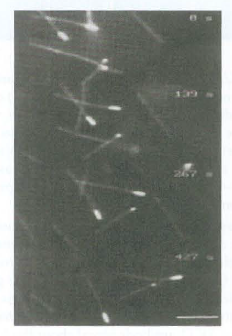

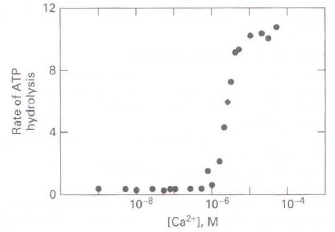
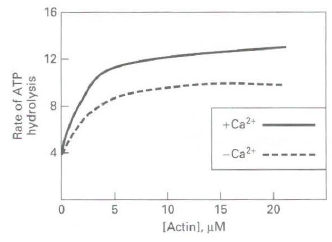
![16 12 +Ca2- -Ca2+ 10 15 [Actin], uM Rate of ATP hydrolysis](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1523/7/0/6/3225ad1e9d2629e61523706286272.jpg)