Question: 1. A metal with unknown identification, M, was found to react with nitric acid with the following stoichiometry: 2 HNO3(aq) + M(s) M(NO3)2(aq) +
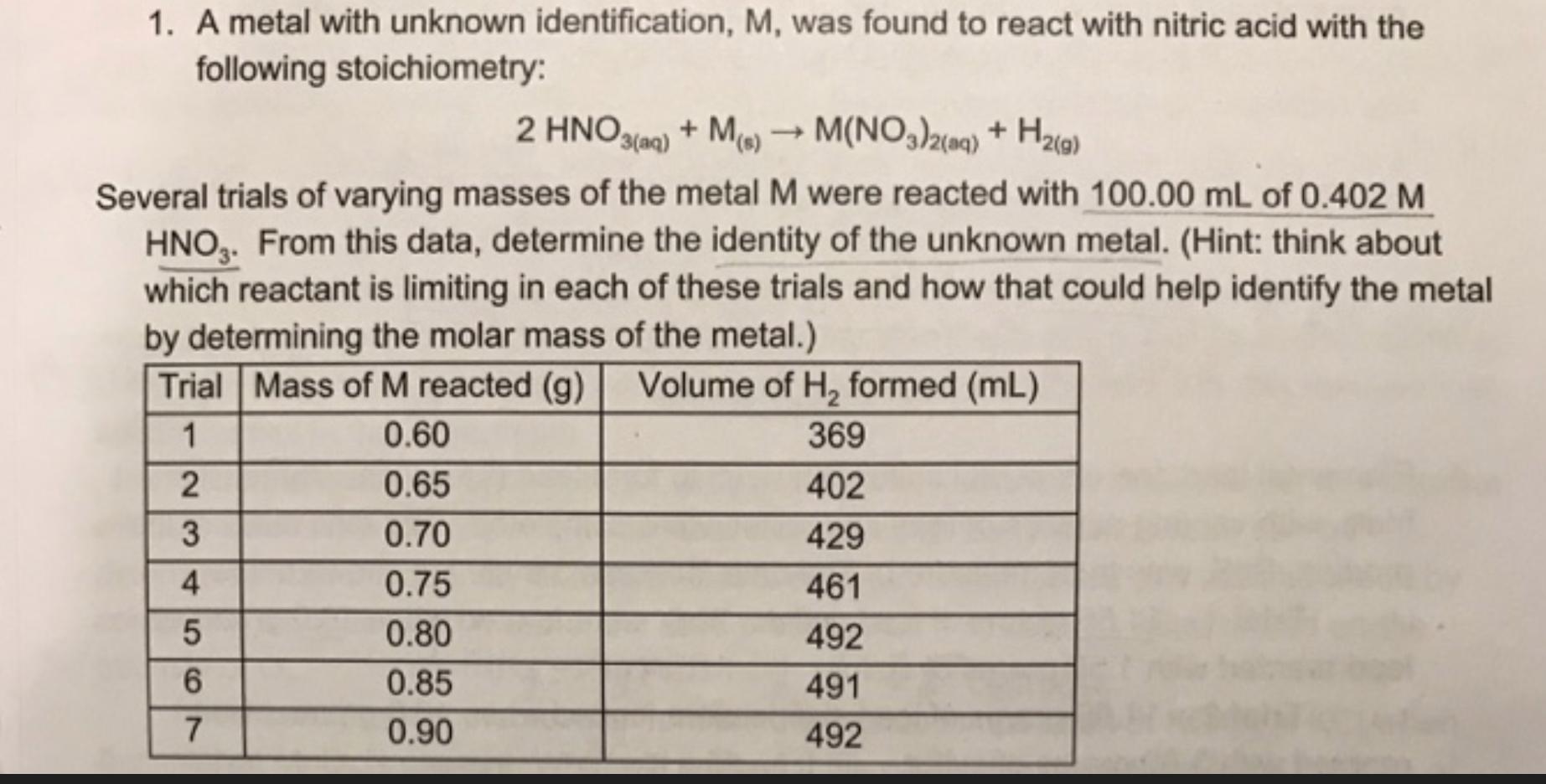
1. A metal with unknown identification, M, was found to react with nitric acid with the following stoichiometry: 2 HNO3(aq) + M(s) M(NO3)2(aq) + H2(9) Several trials of varying masses of the metal M were reacted with 100.00 mL of 0.402 M HNO3. From this data, determine the identity of the unknown metal. (Hint: think about which reactant is limiting in each of these trials and how that could help identify the metal by determining the molar mass of the metal.) Trial Mass of M reacted (g) Volume of H, formed (mL) 1 0.60 369 0.65 402 0.70 429 0.75 461 0.80 492 0.85 491 0.90 492 234567
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Answer 10000 mL of 0402 M As the metal concentration is ... View full answer

Get step-by-step solutions from verified subject matter experts


