Question: 2. n-butane is converted to isobutane in a continuous isomerization reactor that operates isothermally at 160C. The feed to the reactor contains 300mol/h of 90mole%n-butane,
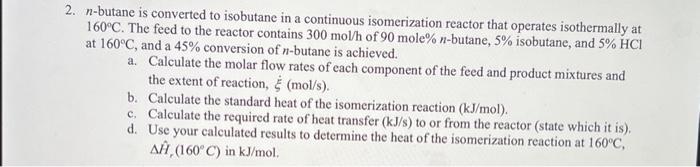
2. n-butane is converted to isobutane in a continuous isomerization reactor that operates isothermally at 160C. The feed to the reactor contains 300mol/h of 90mole%n-butane, 5% isobutane, and 5%HCl at 160C, and a 45% conversion of n-butane is achieved. a. Calculate the molar flow rates of each component of the feed and product mixtures and the extent of reaction, (mol/s). b. Calculate the standard heat of the isomerization reaction (kJ/mol). c. Calculate the required rate of heat transfer (kJ/s) to or from the reactor (state which it is). d. Use your calculated results to determine the heat of the isomerization reaction at 160C, H^r(160C) in kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


