Question: (20 points) Consider a binary liquid mixture of 20 mole% species 1 in species 2 at 25C and 30kPa. The fugacity of pure species 1
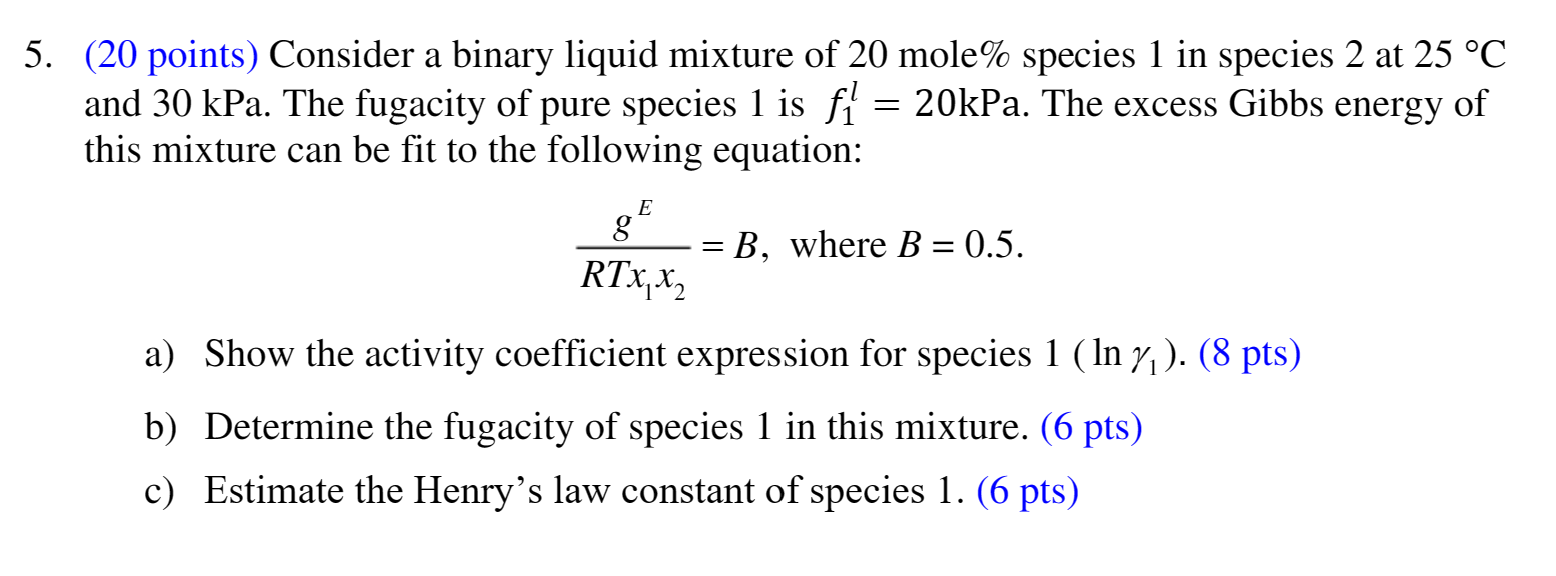
(20 points) Consider a binary liquid mixture of 20 mole\% species 1 in species 2 at 25C and 30kPa. The fugacity of pure species 1 is f1l=20kPa. The excess Gibbs energy of this mixture can be fit to the following equation: RTx1x2gE=B,whereB=0.5. a) Show the activity coefficient expression for species 1(ln1).(8pts) b) Determine the fugacity of species 1 in this mixture. (6 pts) c) Estimate the Henry's law constant of species 1. (6 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


