Question: 41. Use the given standard reduction potentials to determine the reduction potential for this half-reaction. MnO, (aq) + 3 + 4H* MnO2(s) + 2H,00 Reaction
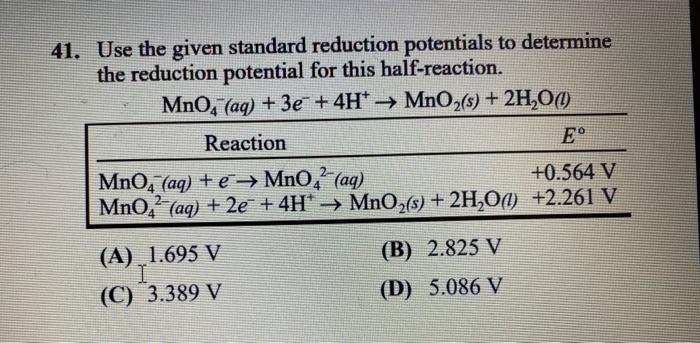
41. Use the given standard reduction potentials to determine the reduction potential for this half-reaction. MnO, (aq) + 3 + 4H* MnO2(s) + 2H,00 Reaction MnO(aq) + MnO,- (aq) +0.564 V MnO,- (aq) + 2e + 4H* MnO2(s) + 2H2O() +2.261 V (A) 1695 V I (C) 3.389 V (B) 2.825 V (D) 5.086 V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


