Question: Use the References to access important values if needed for this question. The rate of the elementary reaction NO+CH3OCH3HNO+CH3OCH2 has been studied as a function
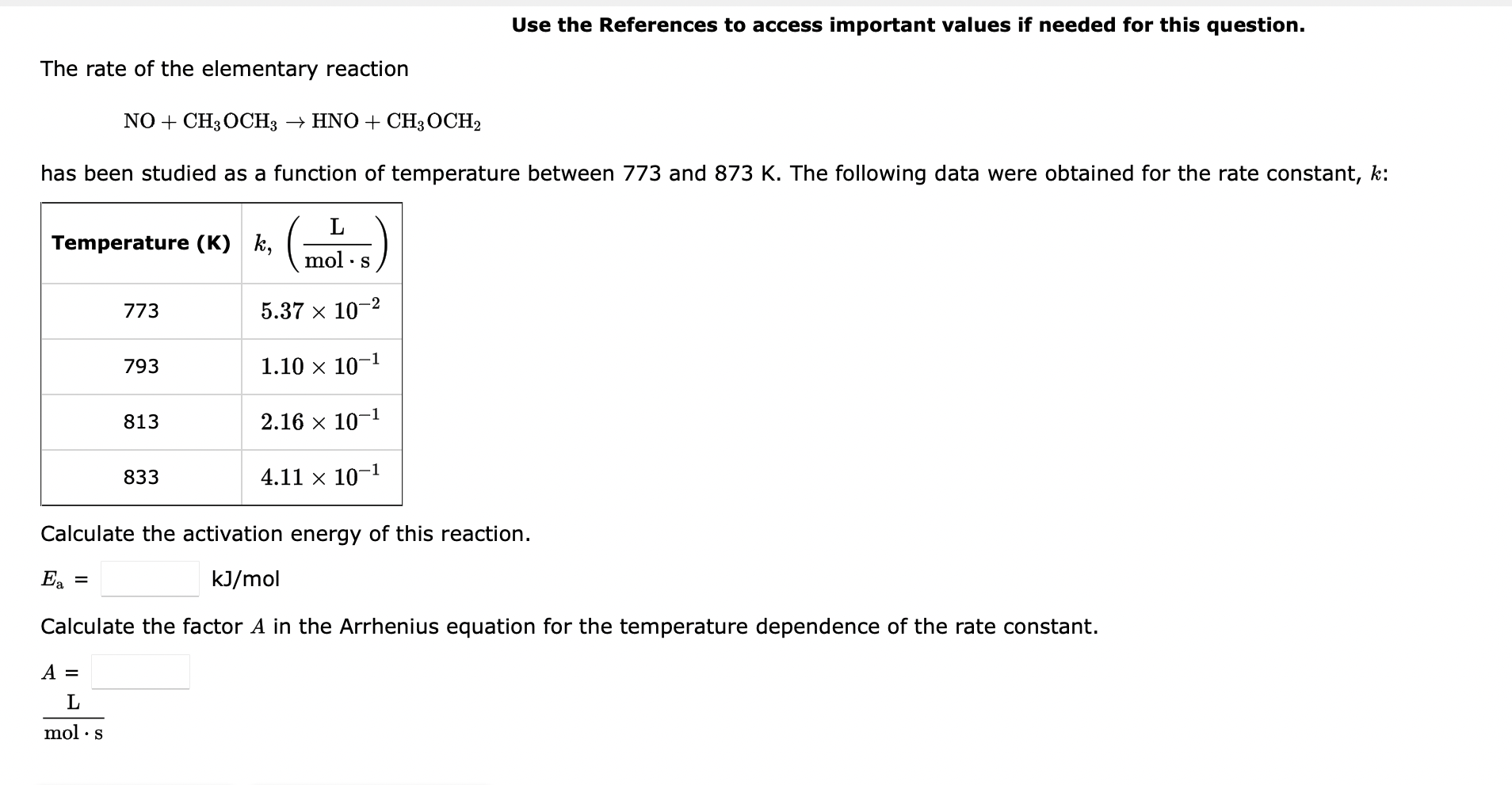
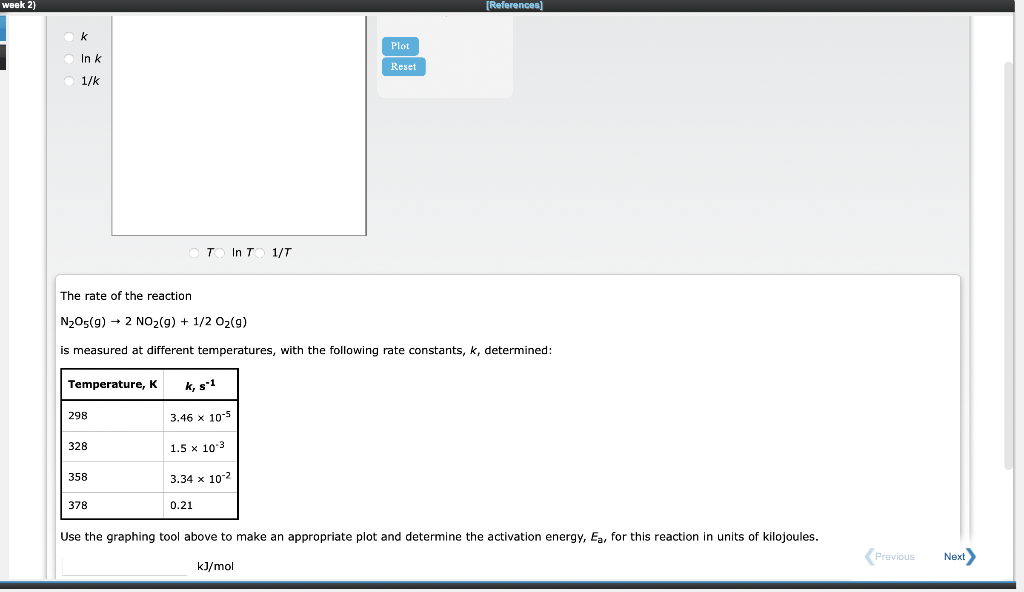
Use the References to access important values if needed for this question. The rate of the elementary reaction NO+CH3OCH3HNO+CH3OCH2 has been studied as a function of temperature between 773 and 873K. The following data were obtained for the rate constant, k : Calculate the activation energy of this reaction. Ea=kJ/mol Calculate the factor A in the Arrhenius equation for the temperature dependence of the rate constant. A=molsL The rate of the reaction N2O5(g)2NO2(g)+1/2O2(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


