A van der Waals gas is going through a JouleThomson process that keeps the enthalpy H constant.
Question:
A van der Waals gas is going through a Joule–Thomson process that keeps the enthalpy H constant. A van der Waals gas in characterised by the following equations of state,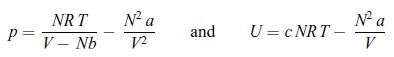
and the amount of gas is constant, i.e. N = const. Use the condition dH = 0 in order to obtain an expression for the derivative dT/dV. Determine the temperature T0 at which this derivative changes sign.
Transcribed Image Text:
P = NRT V- Nb N² a 12 and U = CNRT - N² a V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
To find the expression for the derivative dTdV we first need to calculate the enthalpy H using the g...View the full answer

Answered By

Nicholas Odongo
As an academic writer, I am well-versed in the necessary skills required for successful creation and delivery of written assignments. This includes performing research to find relevant and interesting facts and information, formulating texts following prescribed editorial and formatting guidelines, and communicating with clients to clarify any expectations related to the assignment. Additionally, I am adept at informing the client of any difficulties that arise during the course of the assignment, ensuring that the texts are properly researched and original, proofreading the work for errors, and finally submitting it via the requisite channels. All of these skills serve to ensure that the assignments are of the highest quality and meet the client's expectations.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet
Question Posted:
Students also viewed these Engineering questions
-
For a Van der Waals gas find: (a) The equation of the adiabatic curve in the variables T, V; (b) The difference of the molar heat capacities Cp = Cv as a function of T and V.
-
A liquid mixture containing 70.0 mol of n -pentane and 30.0 mol of n -hexane initially at 46?C is partially vaporized at P = 1 atm in a single-stage distillation apparatus (Rayleigh still). The heat...
-
Making use of the result obtained in the foregoing problem find at what temperature the isothermal compressibility u of a Van der Waals gas is greater than that of an ideal gas. Examine the case when...
-
A vessel is in the form of an inverted cone. Its height is 8 cm and the radius of its top, which is open, is 5 cm. It is filled with water up to the brim. When lead shots, each of which is a sphere...
-
Aaron Lynch Company has the following balances in selected accounts on December 31, 2014. Service Revenue ........$40,000 Insurance Expense...... 2,700 Supplies Expense ....... 2,450 All the accounts...
-
Spreadsheets contain pitfalls for the unwary. (a) If cell A1 contains the value 5, what would you expect the formula = A1^2+A1^2 to give? (b) Using Excel, enter 5 in cell A1, enter = A1^2+A1^2 in...
-
October 19, 1987, is an infamous day in financial history, better known as "the Black Monday of 1987." On that single day, the Dow Jones Industrial Average (DJIA) dropped by \(22.61 \%\), and other...
-
Gary McKnight is evaluating a business opportunity to sell grooming kits at dog shows. Gary can buy the grooming kits at a wholesale cost of $ 32 per set. He plans to sell the grooming kits for $ 62...
-
During Nixon's administration, his attempts to curb inflation were met with challenges. His economic advisors gave advice based on what the federal government usually did, either increase or decrease...
-
Heat pipes are devices used to transfer heat over a certain distance. A typical heat pipe looks like a metal rod, but modern versions, which are used for example to cool the hottest part of a phone,...
-
The Lenoir cycle is a model for the operation of a combustion engine patented by Jean Joseph Etienne Lenoir in 1860 (Figs. 7.2 and 7.3). This idealised cycle is defined y three reversible processes: ...
-
Compute the mass fractions of liquid in the following refractory materials at 1600C (2910F): (a) 6 wt% Al2O3-94 wt% SiO2 (b) 10 wt% Al2O3-90 wt% SiO2 (c) 30 wt% Al2O3-70 wt% SiO2 (d) 80 wt% Al2O3-20...
-
consider the following and state whether you would begin your research in state or federal sources. Can a client who filed bankruptcy five years ago file for bankruptcy again?
-
Identify three steps in the cash handling process where you would use interviewing to gather evidence. What questions would you ask?
-
Amazon Inc. a. Overall vision for the company - where do you want to take the company? What kind of company do you want it to be? What is the logic that holds your portfolio of businesses together?...
-
1. Home Equity. What is home equity? Describe how home equity loans work. 2. New Cars. Explain the advantages and disadvantages of buying a new car instead of a used car. 3. Student Loan Deferment....
-
Using Quinn s seven factors, please prepare a document to predict, uncover, and prepare for any and all eventualities related to these seven factors. This includes getting clarification on questions...
-
Refer to the Forbes list of the 40 best-paid chief executive officers, Exercise. The salary and age of each CEO are saved in the FORBES40 file. a. Use a graph to portray the relationship between a...
-
Archangel Corporation prepared the following variance report. Instructions Fill in the appropriate amounts or letters for the question marks in the report. ARCHANGEL CORPORATION Variance...
-
Use the systematic treatment of equilibrium to find the concentrations of the major species in a saturated aqueous solution of LiF. Consider these reactions: (a) Initially, set the ionic strength to...
-
Heterogeneous equilibria and calcite solubility. If river water in Box 7-2 is saturated with calcite (CaCO 3 ), [Ca 2+ ] is governed by the following equilibria: (a) From these reactions, find the...
-
Using activity coefficients correctly, find the pH of 1.0 10 -2 M NaOH.
-
Drone Berhad wants to do a stock split of 5 to 1. Given below is the partial equity account for Drone Berhad: Partial Equity Account for Drone Berhad (RM) Common Stock @ RM2 Premium Retained Earnings...
-
Update Knowledge of the Events Industry. ASSESSMENT INSTRUCTIONS While being observed by your assessor, you are to use information identified and opportunities to update knowledge of the events...
-
Review the text and course materials for this week and respond to the following questions: Explain the difference between positional and personal power in leadership. Give examples of a leader or...

Study smarter with the SolutionInn App


