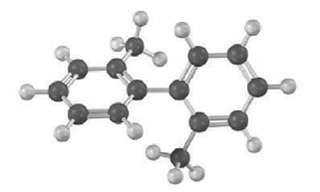
The following molecular model of a dimethyl-substituted biphenyl represents the lowest-energy conformation of the molecule. Why are
Question:
The following molecular model of a dimethyl-substituted biphenyl represents the lowest-energy conformation of the molecule. Why are the two benzene rings tilted at a 63o angle to each other rather than being in the same plane so that their p orbital?s can overlap? Why doesn?t complete rotation around the single bond joining the two rings occur
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
In the lowestenergy conformation of the biphenyl the aromatic rings are ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The following molecular model represents a tetrahedral intermediate resulting from addition of a nucleophile to an aldehyde or ketone. Identify the reactants, and write the structure of the final...
-
The following molecular model is a representation of para-aminobenzoic acid (PABA), the active ingredient in many sunscreens. Indicate the positions of the multiple bonds. And draw a skeletal...
-
The following molecular model is that o a carbocation. Draw two resonance structures for the carbocation, indicating the positions of the doublebonds.
-
Suppose school records reveal that historically, 10 % of the students in Milton High School have dropped out of school. What is the probability that more than two students in a class of 30 will drop...
-
What are the guidelines regarding the deductibility of luxury water travel (e.g., cruise ships) for business purposes? IRS Publication 463.
-
Predict the product of the Dieckmann cyclization that occurs when each of the following compounds is treated with sodium ethoxide. (a) (b) (c) OEt LOET Eto OEt
-
The time to prepare a slide for high-throughput genomics is a Poisson process with a mean of two hours per slide. What is the probability that 10 slides require more than 25 hours to prepare?
-
Your friendly foreign exchange trader has given you the following currency cross rates. The quotes are expressed as units of the currency represented in the left-hand column per unit of currency...
-
Required information [The following information applies to the questions displayed below.] George and Wanda received $30,500 of Social Security benefits this year ($11,700 for George; $18,800 for...
-
Refer to the Report of Independent Auditors of Unilever N. V. and Unilever PLC, signed on 5 March. Required: Identify the features in the above audit report that are unique to an MNC.
-
Draw the product from reaction of each of the following substances with (i) Br2, FeBr3 and (ii) CH3COCl,AlCl3. (b) (a)
-
How would you synthesize the following compound starting from benzene? More than one step isneeded.
-
One long solenoid is placed inside another solenoid. Both solenoids have the same length and the same number of turns of wire, but the outer solenoid has twice the diameter of the inner solenoid....
-
3. Are certain styles or conventions of art - such as the use of hierarchical scale, reliance on certain types of shapes and forms, or certain ways of representing people and nature - more likely to...
-
If a not-for-profit organization uses the restricted fund method to account for contributions and receives a restricted contribution for which it does not have a corresponding restricted fund, how...
-
(A) (B) (C) (D) House Leadership Roles The Speaker of the House is the presiding officer who guides the passage of legislation favored by his or her party. The House majority leader is the floor...
-
4) Johnny loves to go to Taco Tuesdays at Rosa's Cafe. Each week on Taco Tuesday Johnny consumes tacos and sodas according to the following utility function: = U(t,s) 20t t + 18s - 3s - a. How many...
-
According to the Center for Responsive Politics, Houseand Senate candidates who are the top campaign spenders usually winelections as shown in the chart below. This money comes from fourmain sources,...
-
What led Congress to the realization that the traditional common-law transactional approach to crime was inadequate to deal with organized crime?
-
Uniform electric field in Figure a uniform electric field is directed out of the page within a circular region of radius R = 3.00 cm. The magnitude of the electric field is given by E = (4.50 x 10-3...
-
Evaluate the difference for the ideal and van der Waals gases, and for a gas that obeys the virial equation of state. (3) - (0) P V
-
Show the products of thesereactions: Br ELOH a) CH3CH,CH + CH;CH,0 I 1) Na 2) CH,I CH,CH,CH,CH, NaOH b) c) ELOH CH3
-
Diphenhydramine can also be synthesized by heating bromo diphenyl methane with the amino alcohol shown here. Offer a reason why the oxygen, rather than the nitrogen, of this compound acts as the...
-
Explain which route would provide a better synthesis of theseethers: CH3 CH, CH3 CHI + CH,CO a) CH.O + CH,CCI CH3 CH,COCH3 H3 CH3 CH, CH,OCHCH,CH; CH,Br CH,0 Br + CH;CHCH,CH3 b) + CH;CHCH,CH,
-
Ruzgar Inc., bonds were selling at $1,040 a year ago. A friend of yours invested into this bond and just received a $100 coupon payment. She sold the bond today for $1,200.If the inflation rate for...
-
Explain possible advantages and disadvantages of PSL's current international trading strategy (subsidiary in China, export & import and online presence).
-
In November 2020 you entered into four May 2021 long futures contracts for crude oil (1,000 barrels per contract) for $65.00 per barrel. If the futures price was $70.00 per barrel on December 31,...

Study smarter with the SolutionInn App


