Question: 2. ( 6 points) The uranyl tris-carbonate ion from the previous reaction can be treated with a strong base to precipitate Na2U2O7. Write a balanced

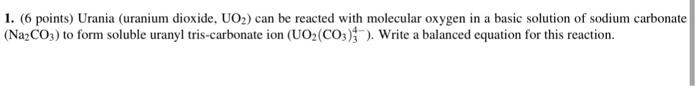
2. ( 6 points) The uranyl tris-carbonate ion from the previous reaction can be treated with a strong base to precipitate Na2U2O7. Write a balanced equation for this reaction. 1. (6 points) Urania (uranium dioxide, UO2 ) can be reacted with molecular oxygen in a basic solution of sodium carbonate (Na2CO3) to form soluble uranyl tris-carbonate ion (UO2(CO3)34). Write a balanced equation for this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


