Question: 28 Answer options: 10-31 or none ----> Consider the reaction below: 2 Fe(NO3)3 (aq) + 3 Mg(OH)2(aq) 2 Fe(OH)3(s) + 3 Mg(NO3)2(aq) (OR 2 Fe(NO3)3
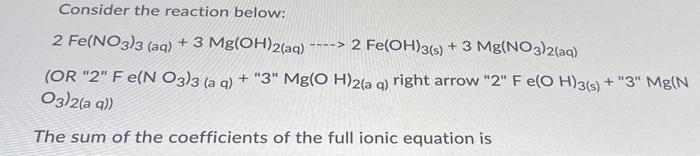
----> Consider the reaction below: 2 Fe(NO3)3 (aq) + 3 Mg(OH)2(aq) 2 Fe(OH)3(s) + 3 Mg(NO3)2(aq) (OR "2" Fe(NO3)3 (a q) + "3" Mg(OH)2(a q) right arrow "2" Fe(OH)3(s) + "3" Mg(N O3)2(a q)) The sum of the coefficients of the full ionic equation is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


