![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


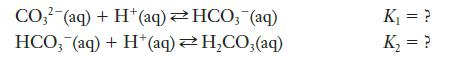
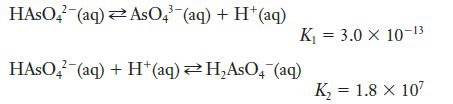
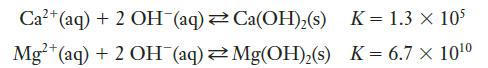
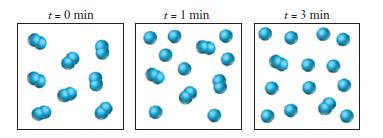
![Exposure Time, t (days) 0 7 14 21 28 35 42 49 56 [Picloram] (mol L-) 4.14 x 10-6 3.70 x 10-6 3.31 x 10-6 2.94](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/9/0736564411159e5e1701069072171.jpg)
![t1/2 1 k[A]o](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/8/919656440775170d1701068918168.jpg)
![Experiment 1 2 Initial [NO5] (mol L-) 3.0 10- 9.0 10- Initial Rate of Reaction (mol L s ) 9.0 10-7 2.7 x](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/0/26965641eada74a71701060268770.jpg)
![Experiment 1 2 3 Initial [NO] (mol L-) 2.3 x 10-5 4.6 x 10-5 4.6 x 10-5 Initial [03] (mol L-) 3.0 10-5 3.0](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/0/44165641f59b038c1701060440806.jpg)
![Time (s) 0.0 5.0 10.0 15.0 20.0 25.0 30.0 [NO] (mol L-) 3.00 x 10-1 1.97 x 10-2 1.00 10- 7.00 10- 5.20 10-](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/0/741656420852b2ed1701060740232.jpg)
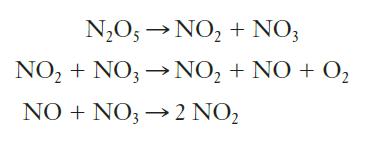


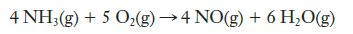
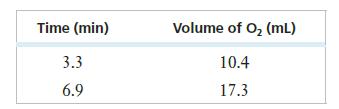

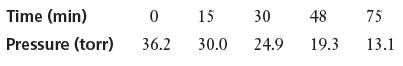
![Rate (mL min-) 0.090 0.178 0.184 [HO] (mol L-) 0.15 0.30 0.15 [KI] (mol L-) 0.033 0.033 0.066](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/6/149656435a5687a41701066148284.jpg)
![[A] (mol L-) 0.127 0.127 0.255 2 A+ 3B C + 2D [B] (mol L-) 0.15 0.30 0.15 Rate = A[C]/At (mol L- s-) 0.033](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/7/33965643a4b2bb681701067338062.jpg)
![NH4+ (aq) + NO (aq) N(g) + 2 HO(l) [NH+] (mol L-) [NO ] (mol L-) Rate = A[N]/At (mol L-s-) 0.0092 0.0092](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/7/44265643ab2a2aa51701067441574.jpg)
![Expt. 1 2 3 4 A + 2B C + 2D Initial [A] (mol L-) 0.10 0.30 0.10 0.20 Initial [B] (mol L-) 0.10 0.30 0.30 0.40](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/7/75365643be9846931701067752182.jpg)
![[NO] (mol L-) 0.002 0.002 0.006 [0] (mol L-) 0.005 0.010 0.005 Rate = A[NO]/At (mol L-s-) 8.0 10-17 1.6](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/7/85965643c53b85d71701067858671.jpg)
![Time, t (min) 0 5 10 15 20 25 30 35 40 [NO5] (mol L-) 0.200 0.171 0.146 0.125 0.106 0.0909 0.0777 0.0664](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/6/9/005656440cda9d1f1701069004447.jpg)