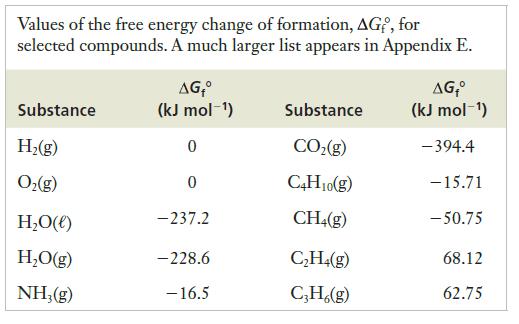
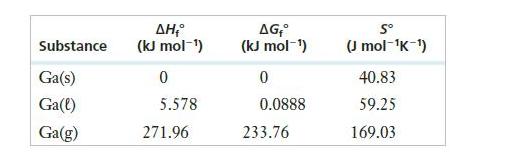
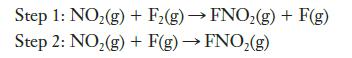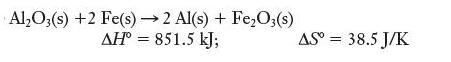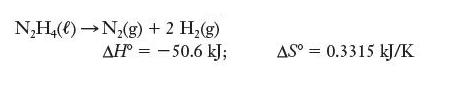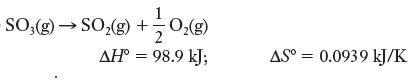![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


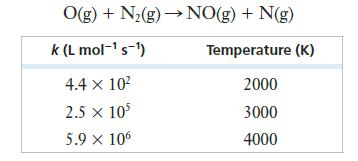
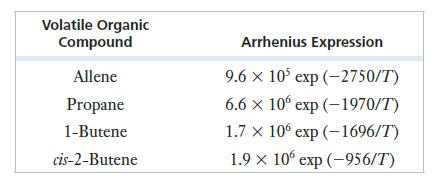
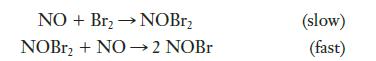
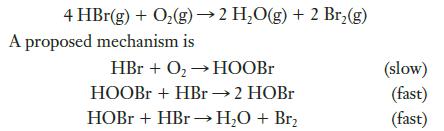

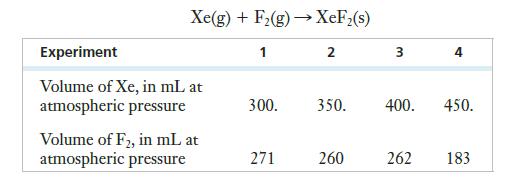
![2 NO(g) + F(g) 2 FNO(g) has the rate law Rate = k[NO][F]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/7/4/96965645819936b91701074968278.jpg)