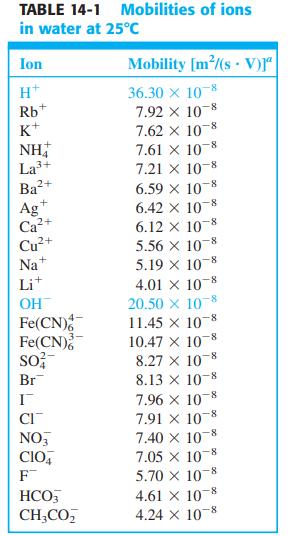
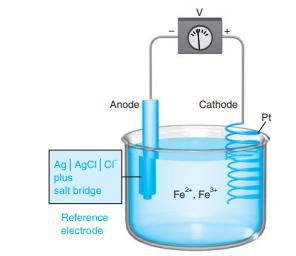
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


.png)
.png)
.png)
-1.png)
-2.png)
.png)
-1.png)
-2.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
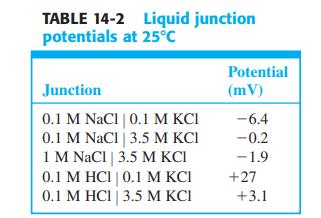
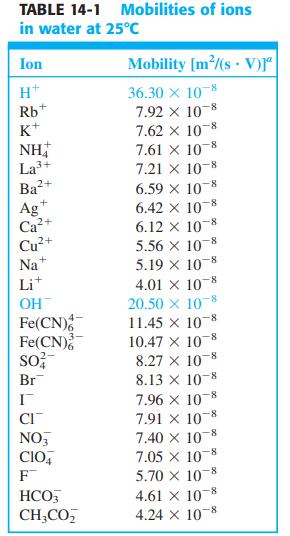
![V Solution a | Solution B [C(B)- C;(a)] RT - In F 2 ziļu,[C(B) - C;(a)] Z,/u; C;(a) Zi E; 2 zilu, C;(B) i](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1591/3/4/2/7015ed9f66db65361591342699519.jpg)